Dental tissue injury and regeneration affects the daily lives of almost everyone. Tissue engineering is emerging as a promising therapy to regenerate missing teeth and dental tissues. The aim of regenerative dental therapies is to restore patients to full oral health. This means restoring normal function to missing or damaged tissue. Regeneration approaches use a combination of scaffolds, stem cells, growth factors, tissue engineering, organ tissue culture, transplantation, and tissue grafting. There are 8 key elements to create and use tissue constructs for tissue regeneration. These will be described in detail in this article.
- •
There are 8 key elements that are needed to create tissue constructs for dental regeneration.
- •
The creation of tissue constructs requires scaffolds to be seeded with stem cells in a controlled environment with growth factors.
- •
The type of stem cells and selection of scaffolds are based on the need to regenerate mucosa, gingiva, pulp or other dental tissue.
Dental tissue injury and regeneration affects the daily lives of almost everyone. People who have missing teeth and dental tissues have difficulty eating and speaking, suffer a lower health-related quality of life, are less likely to be employed, more likely to be depressed, and can suffer emotional problems because of their unhealthy appearance. Dental implants and dentures can be aesthetically and functionally effective and also satisfy patients. But dental implants are not able to completely replace all the functions of natural teeth. Gingiva, skin, and bone can be replaced by transplanting donor tissues, but these therapies are limited by donor shortage, or sometimes the limited regenerative ability of donated tissues. Tissue engineering is emerging as a promising therapy to regenerate missing teeth and dental tissues. A key element of tissue engineering is to generate a functional tissue construct, to replace lost or damaged tissues. Most dentists believe that regenerative dental treatments involving the delivery of stem cells and tissue constructs will become common within the next 10 to 20 years. Most dentists are willing to receive training to be able to provide regenerative endodontic procedures for their patients. Half of dentists already use membranes, scaffolds, or bioactive materials. It was surprising that 55% of dentists were unsure whether regenerative procedures would be successful. The surveys indicate broad support among dentists for using scaffolds and constructs to regenerate tissues, but also the need for strong evidence that demonstrates the reliability of using these new therapies.
Regeneration of dental tissue
The aim of regenerative dental therapies is to restore patients to full oral health. This means restoring normal function to tissue that has been damaged or missing due to trauma, disease, cancer, or a congenital condition. Greenwood and colleagues described regenerative medicine as the “emerging interdisciplinary field of research and clinical applications focused on the repair, replacement, and regeneration of cells, tissues, or organs to restore impaired function resulting from any cause, including congenital defects, disease, trauma, and aging.” Regeneration approaches use a combination of scaffolds, stem cells, growth factors, tissue engineering, organ tissue culture, transplantation, and tissue grafting. There are 8 key elements to create and use tissue constructs for tissue regeneration.
-
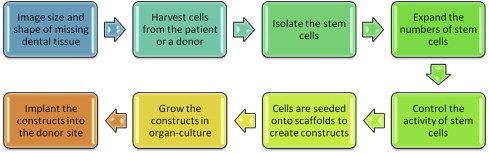
Step #1. The size, shape, functions, and aesthetics of the missing or defective tissue, such as a bone defect, needs to be assessed using cone beam microcomputer tomography and radiographs.
-
Step #2. Stem cells, such as dental pulp stem cells from an exfoliated baby tooth, need to be obtained from the host patient or a donor to serve as the building blocks for tissue regeneration.
-
Step #3. Stem cells account for a very small percentage of the cells within tissues. The stem cells must be identified using surface markers and be isolated from all the donated cells using fluorescent cell sorting.
-
Step #4. Millions of stem cells are needed to create functional tissues; this requires that they be expanded using cell culture.
-
Step #5. The activity of the stem cells must be controlled by growth factors during cell culture to ensure that the stem cells differentiate into a useful cell type (eg, bone or periodontal ligament).
-
Step #6. Cells grown in culture lack a 3-dimensional scaffold necessary to function and have the correct size and shape to generate a tissue; therefore the cells need to be seeded onto a scaffold to form a tissue construct that gives the cells the characteristics of a tissue, such as dental pulp stem cells seeded onto polymer and collagen scaffolds to generate replacement pulp tissue.
-
Step #7. The tissue construct is maintained in cell culture until a functional tissue is generated.
-
Step #8. The tissue construct is grafted or implanted into the donor site, where the regenerated tissue is required.
These steps to create and use tissue constructs are shown in Fig. 1 .
Dental regeneration
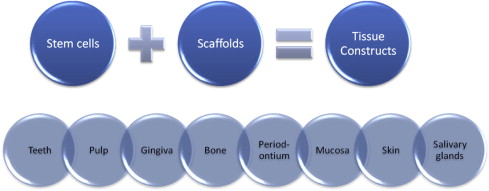
Dental regeneration can be defined as the process in people by which specialized dental tissues are replaced by the recruitment, proliferation, migration, and differentiation of dental stem cells. It is important that the newly regenerated tissues recapitulate the architecture and function of the missing or damaged dental tissue. However, ideal reconstructive goals, such as a complete return to original clinical form and function, are frequently not completely achieved. Regeneration can occur more readily in some tissue types compared with others (eg, the oral mucosa can readily regenerate without scarring, while most other dental tissues heal by granulation tissue, which can eventually form fibrous scar tissue). Dental tissue regeneration depends on the activity of progenitor cells or stem cells to be seeded within a scaffold to generate a new tissue construct (eg, artificial skin that can serve as a scaffold for the regrowth of dermal tissue using the hosts own cells). Another example is freeze-dried bone, which provides a scaffold for the host’s own osteoblasts to regenerate bone defects. By combining different types of stem cells with different types of scaffolds and controlling the cell culture and tissue engineering conditions, the outcome of the construct can be altered to create various types of dental tissues, from teeth to salivary glands, shown in Fig. 2 .
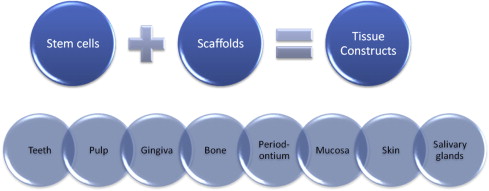
Dental regeneration
Dental regeneration can be defined as the process in people by which specialized dental tissues are replaced by the recruitment, proliferation, migration, and differentiation of dental stem cells. It is important that the newly regenerated tissues recapitulate the architecture and function of the missing or damaged dental tissue. However, ideal reconstructive goals, such as a complete return to original clinical form and function, are frequently not completely achieved. Regeneration can occur more readily in some tissue types compared with others (eg, the oral mucosa can readily regenerate without scarring, while most other dental tissues heal by granulation tissue, which can eventually form fibrous scar tissue). Dental tissue regeneration depends on the activity of progenitor cells or stem cells to be seeded within a scaffold to generate a new tissue construct (eg, artificial skin that can serve as a scaffold for the regrowth of dermal tissue using the hosts own cells). Another example is freeze-dried bone, which provides a scaffold for the host’s own osteoblasts to regenerate bone defects. By combining different types of stem cells with different types of scaffolds and controlling the cell culture and tissue engineering conditions, the outcome of the construct can be altered to create various types of dental tissues, from teeth to salivary glands, shown in Fig. 2 .
History of dental regeneration
From the beginnings of dentistry in Egypt almost 5000 years ago, dentists have been seeking improved procedures to generate replacement teeth, and to regenerate dental tissues for their patients. In 1952, BW Hermann was reported to have made one of the first attempts at dental tissue regeneration. Herman used calcium hydroxide (calxyl) to promote dentin bridge formation for vital pulp therapy following partial pulp amputation. In 1961, Nygaard-Ostby established a blood clot to use as a scaffold to revascularize tissue within the root canals of teeth ( Fig. 3 ). During recent decades, many more dental procedures and scaffolds have been developed to promote dental tissue regeneration, especially in the field of periodontics to guide bone and tissue regeneration. The concepts of guided tissue and bone regeneration (GTR/GBR) were first published by Melcher in 1976, who outlined the necessity of using barrier membranes to exclude unwanted cell lines from healing sites to allow tissue growth. The first clinical case reports of GTR/GBR were presented by Nyman and colleagues through the intraoral application of Millipore bacterial filters composed of a cellulose acetate as the first barrier membranes used to achieve regeneration of periodontal tissues as a direct alternative to surgical resection procedures to reduce periodontal pocket depths.
Next came the introduction of platelet-rich plasma (PRP) to use as a natural fibrin scaffold as part of oral surgery by Whitman and colleagues in 1997. PRP is thought to activate platelets and release growth factors to enhance wound healing. PRP became more popular after Marx and colleagues demonstrated that combining PRP with autogenous bone in mandibular continuity defects resulted in significantly faster radiographic maturation and histomorphometrically denser bone regeneration. PRP has also been used in the field of regenerative endodontics, with the recent case report of Torabinejad and Turman, which demonstrated the merits of PRP as a natural scaffold for pulp revascularization.
Scaffolds
People and animals have a natural scaffold that surrounds cells and provides structural support for the formation and maintenance of tissues and organs. The scaffold is mainly composed of extracellular matrix proteins (ECMPs). The key ECMPs are collagen, vitronectin, fibronectin, and laminin, which provide cells with anchorage, sequestration of growth factors, and signal cells to migrate, differentiate, and proliferate through integrin receptor-mediated signaling pathways. ECMPs have important roles in dental regeneration. Laminin promotes odontoblast differentiation, and a recent study by Howard and colleagues demonstrated that it is an important factor in dental pulp stem cell migration. Fibronectin has been shown to increase ameloblast growth and differentiation, while vitronectin provides a structural framework. Collagen is the predominant structural component of all tissues and has been observed to immobilize growth factors to regulate cell proliferation and differentiation.
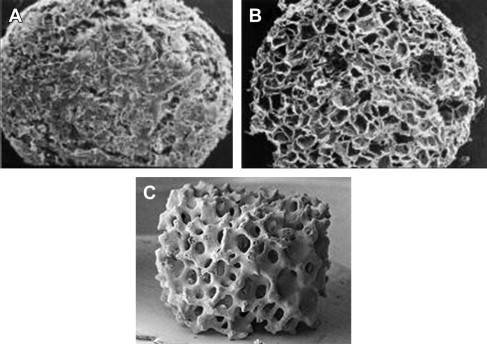
Natural ECMP scaffolds have varying chemical and physical characteristics which contribute to the specific functions of the tissue in which they reside. Scaffolds for tissue engineering have been created with a range of physical properties; these include porosity, pore size, weight, and hydration capacity, as shown in Table 1 . A high porosity and sufficient pore size are necessary to facilitate cell seeding and diffusion of cells and nutrients throughout scaffold. The other important properties are biocompatibility and biodegradability. Some scaffolds are permanent, while for other scaffolds, they need to be absorbed by the surrounding tissues to avoid interfering with the regenerated tissue. The rate of degradation should coincide with the rate of tissue formation. The latest generations of scaffolds have been engineered to have ideal properties and functional customization: injectability, synthetic manufacture, biocompatibility, nonimmunogenicity, transparency, nano-scale fibers, low concentration, and high resorption rates. Scaffolds for tissue engineering can be created from synthetic materials such as polymers, similar to absorbable surgical sutures, or by collagen, a natural ECMP scaffold material, or by calcium phosphate. The architecture of these scaffolds is shown in Fig. 4 .
| Scaffold Type | Average Dimensions | Hydration Capacity | Porosity (Pores Per Linear Inch) | Average Pore Size | Weight/Wet Weight |
|---|---|---|---|---|---|
| Polymer | 5 × 4 mm 0.039 cm 3 | 30 mL | 120−/+20 | 100–200 mm | 5.2 mg/32 mg |
| Collagen | 4.5 × 4.2 mm 0.039 cm 3 | 25 mL | 120−/+20 | 100–200 mm | 3.5 mg/45 mg |
| Calcium Phosphate | 5 × 3 mm 0.058 cm 3 | 30 mL | 60−/+10 | 200–400 mm | 45 mg/99 mg |

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


