
13

Advances in Radiation Therapy for Salivary Gland Neoplasms
Several chapters on the management of major and minor salivary gland cancers have been written over the last decade, providing the surgeon and radiation oncologist with guidelines to treating these neoplasms. What has changed, then, that would require additional insight? The vast experience published in treating these neoplasms is retrospective in nature, and few prospective trials have been conducted. As a result, drawing firm conclusions from the literature should be made with caution because of the heterogeneity of the patient population, tumor sites, histologies, stages, and reasons for choosing one therapy over another.
The major changes in management have been the adoption, albeit slow, in both academic and community cancer centers across the United States of a new philosophy toward a multidisciplinary team approach. In the field of radiation oncology, rapid advancements in imaging technology—magnetic resonance imaging (MRI), spiral computed tomography (CT) scans, and positron emission tomography (PET) scans—fusion and computer planning software, and treatment delivery technology have been remarkable. Highly complex, conformal radiotherapy plans such as intensity modulated radiation therapy (IMRT) selectively distribute high doses to the intended target while limiting doses to critical normal structures. More complex radiotherapy is now being investigated, where biologic imaging information from PET and radiobiologic principles of tumor and normal tissue responses are being incorporated into the algorithms that determine differential beam intensity depending on the type of tumor, its unique biologic growth rate, and its position in the body relative to other normal tissues. This has significant implications for improving the therapeutic ratio. For unresectable tumors, the introduction of newer chemotherapy agents that have activity against salivary tumors provides a chance to improve local control rates for tumors that typically have had a poor prognosis by combining with conventional radiotherapy in a concurrent fashion. Alternative forms of ionizing radiation that differ from conventional photon radiation (e. g., charged particle therapy) have been and are currently under investigation that have both radiobiologic and physical characteristics that may be advantageous in improving local control. As the intensity of the therapy is increased, the potential for more acute and late side effects increases. The introduction of radioprotectors in the last 5 years during radiotherapy may reduce xerostomia and mucositis during treatment and prevent late side effects that affect the patient’s quality of life.
 General Radiation Therapy Management
General Radiation Therapy Management
Indications for Primary Radiation Therapy: Major Salivary Glands
The major salivary glands are paired glands, have orderly lymphatic drainage patterns, and almost never require management of the opposite side or contralateral neck. The reasons to treat malignant salivary glands with radiotherapy in the absence of surgery include the following: (1) when the surgeon determines the tumor to be unresectable, (2) if surgery is technically feasible but medically prohibitive as a result of patient comorbidities or poor functional status, (3) in the setting of unresectable recurrent disease, and (4) if surgery is technically feasible but would result in unacceptable functional and/or cosmetic outcome.
The perception in the head and neck surgical community that partially resected or unresected parotid neoplasms are radioresistant is unfounded and is not supported in the available literature. The majority of reported data, however, is from small, single institutional experiences that often lump different histologies, stages, and sites (major vs minor salivary) and recurrent versus primary unresectable disease into their outcome analysis. Alternative strategies and outcomes to improve local control over conventional radiotherapy will be highlighted in this chapter.
Indications for Adjuvant Radiation Therapy: Major Salivary Glands
The indications for adjuvant radiation therapy are multifactorial and include (1) positive microscopic margins, (2) deeply infiltrative tumors beyond the parotid bed, (3) perineural invasion, (4) high grade or poorly differentiated tumors, (5) recurrent disease, (6) piecemeal resection despite negative margins, and (7) when the surgeon feels uncomfortable about the gross surgical findings despite negative margins. Failure to at least consider adjuvant radiotherapy for the above reasons increases the risk of local recurrence and reduces the ability to gain local control in the future should a recurrence occur. It further jeopardizes the preservation of the facial nerve and its branches, which in turn can lead to devastating functional and cosmetic consequences.
Indications for Elective Treatment of the Neck: Major Salivary Glands
The indications for elective treatment of the neck have been defined through retrospective patterns of failure after large surgical series.1,2 In general, high-grade, poorly differentiated tumors, regardless of histology, and large tumors (>4 cm) have a high rate of occult ipsilateral neck metastases and require consideration for adjuvant treatment. The risk of contralateral occult neck metastases is too low to warrant treatment even in the setting of multiple ipsilateral nodal metastases.
Advances in Radiation Treatment Planning
Recent publications for major and minor salivary gland neoplasms have incorporated complex treatment-planning methods such as CT-based image acquisition with advanced contouring tools, sophisticated computer software for treatment planning, three-dimensional conformal radiotherapy (3D-CRT), and, most recently, intensity modulated radiation therapy, which provide tight dose gradients that spare surrounding normal structures, and the use of sophisticated automated beam shaping devices such as multileaf collimators (MLCs) to deliver multiple beams in rapid succession.
Advances in Target Delineation
A more comprehensive understanding by the radiation oncologist of head and neck anatomy, as well as careful understanding of nodal sites at risk, is required now that a majority of treatment planning is based on anatomical information obtained from CT scans. In other words, we have to think like surgeons. The selection of nodal sites at risk has for the most part been an arbitrary exercise based on the individual radiation oncologist’s perception of risk. Primary site, involved nodal, and high-risk elective nodal target volumes can now be delineated with the help of published reference guides to assist in the location of lymph node levels, in relation to CT-based anatomical landmarks.3–5 In 2004, a consensus was achieved by a joint surgical-radiation oncology group to arrive at an agreement of anatomical nodal groups, their percent risk of harboring microscopic disease, and their locations using recognizable anatomical reference points on CT.6
Advances in Simulation and Image Acquisition
Prior to the introduction of CT simulators, treatment planning for parotid tumors was performed with the use of a conventional fluoroscopic simulator. Treatment was commonly delivered with a two-dimensional approach using either a wedged pair of ipsilateral photons fields or mixed photon electron fields in an enface technique. Current spiral CT simulators allow for the rapid acquisition of selected head and neck anatomy and provide a platform for three-dimensional reconstruction. Initial CT image acquisition for parotid neoplasms is generally performed with the patient’s head in the supine position in a rigid immobilization mask to prevent motion and limit treatment setup error (Fig. 13–1). Intravenous (IV) contrast is not always necessary if a good contrast diagnostic scan was performed at the time of diagnosis. However, if surgery was performed and a delay longer than 6 weeks occurs from the completion of surgery until treatment planning, then IV contrast is encouraged to reduce the risk of missing a new lymph node metastasis. One advantage of CT simulation is the ability to produce digitally reconstructed radiographs (DRRs) after the patient has left the simulation suite (Fig. 13–2). This leads to an expedited, convenient experience for the patient because DRRs of any field angle can be generated after image acquisition and 3D reconstruction and later viewed and compared for setup accuracy with on-treatment digital port films. No longer does the patient have to endure a prolonged simulation waiting for hard copy films to be produced.
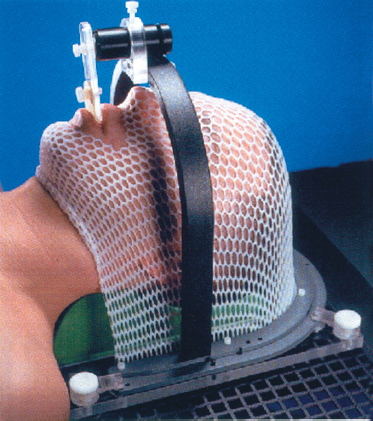
Figure 13-1 Example of rigid head and neck immobilization for three-dimensional conformal radiotherapy (CRT) or intensity modulated radiation therapy (IMRT).
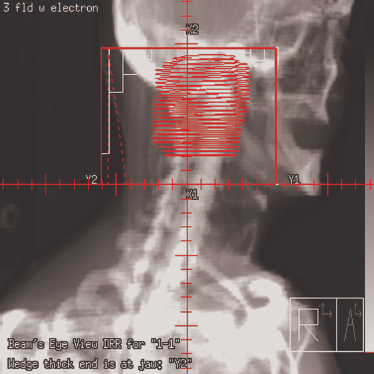
Figure 13-2 Digitally reconstructed radiograph (DRR) of target and normal anatomy with multileaf collimator (MLC) automatic blocking.
Advances in Computer Treatment Planning
Anatomical data acquired in the CT simulator are downloaded into a treatment-planning computer system. At this point, a decision is made to treat with 3D-CRT or IMRT. The physician locates and contours the primary and nodal target volumes for treatment, and normal structures to avoid. Gross tumor and gross nodal disease are delineated on the computer and then expanded to include regions of high-risk microscopic spread. This clinical target volume (CTV) includes both regions adjacent to the primary tumor, as well as nodal regions felt to be at high risk for metastatic spread (Fig. 13–3). The CTV is often not a spherical or symmetrical expansion, and it generally requires a more individualized approach when the CTV overlaps with critical normal structures such as the spinal cord. The critical structures designated by the radiation oncologist take priority over the CTV and cannot be compromised. A final margin is then added to account for potential setup error and either patient or primary target motion called the planning target volume (PTV). Target delineation is performed on the computer planning station with access to 3D-reconstructed images in the axial, coronal, and sagittal planes. Significant advances in fusion software allow other diagnostic imaging modalities such as MRI and more recently PET (Fig. 13–4) to be imported and then overlaid onto to the planning CT for additional anatomical and biologic information. The incorporation of PET scanning for major salivary gland cancers is controversial, given the typical slow tumor growth associated with these neoplasms. However, for more aggressive cancers, they may demonstrate regional or distant sites of metastases that would alter radiotherapy fields or overall management. In case of adenoid cystic cancers or other advanced cases requiring base of skull coverage, MRI fusion can be invaluable. Normal structures are contoured either adjacent or in close proximity to the parotid gland or structures of critical value, such as the spinal cord, temporal lobe, cochlea, lens, and orbit. Organs at risk (OARs) are contoured to differentiate them from the target volume. Normal structures that are critical to life cannot be overdosed and take priority over the PTV. Other important normal structures that are necessary to maintain a reasonable quality of life are contoured, such as the contralateral parotid. One of the most significant side effects of head and neck radiotherapy is xerostomia. Several investigators have studied the tolerance of the parotid and the radiation threshold dose leading to the development of xerostomia. Meaningful parotid sparing should be a goal of any IMRT plan, but not at the expense of PTV coverage. Although estimates of the threshold dose vary, the mean dose delivered to the parotid is predictive of sustained xerostomia. Thus meaningful parotid sparing is achieved when the mean dose does not exceed 26 Gy.7 Other excellent references are available that describe the respective tolerance doses of normal head and neck OARs to irradiation.8
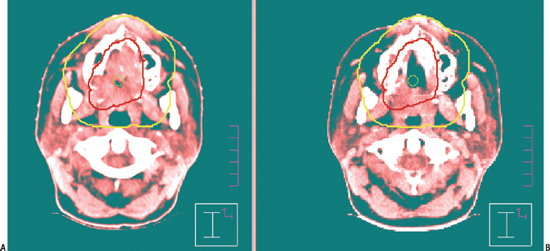
Figure 13-3 Example of an outline of (A) gross (GTV) and (B) clinical (CTV) target volumes for IMRT. This is a 27-yearold female patient who presented with unresectable T4 adenoid cystic carcinoma of the hard palate. She received two cycles of neoadjuvant chemotherapy (carboplatin and a taxane) at the beginning of treatment, began IMRT, and was rescanned at 30 Gy. Significant tumor regression occurred, allowing generation of a second IMRT plan to avoid exceeding tolerance of the optic nerves and chiasm. The base of the skull was included.
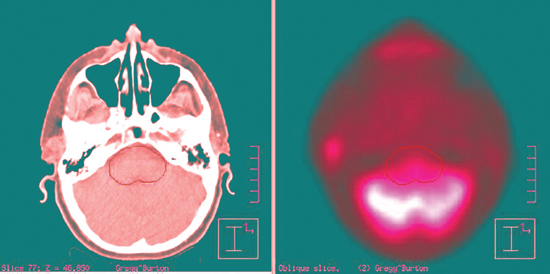
Figure 13-4 Example of the incorporation of positron emission tomography/computed tomography (PET-CT) fusion software for 3D-CRT planning of a parotid mass. The mass can be seen just anterior to the patient’s right ear. The brainstem is outlined to evaluate the dose to that structure.
Intensity Modulated Radiation Therapy
With so many critical normal structures in proximity to the parotid gland, delivering a homogeneous tumoricidal dose to the parotid and nodal levels at risk is a challenge. Additionally, the internal inhomogeneity of the head and neck combined with changes in external contour from vertex to clavicle provides a significant challenge in treatment planning. The development of sophisticated computer planning in the early 1990s led to refinements in conformal beam shaping and delivery, which in turn improved tumor coverage and dose conformality.9 Selection of beam directions and beam shaping was based on 3D images of the target and normal tissues acquired from CT simulation. Three-dimensional conformal radiotherapy, once considered state of the art, is now a standard and mandatory approach to treatment of parotid tumors. The recent development of intensity modulated radiation therapy has brought excitement and promise for even greater target conformality and normal tissue avoidance. IMRT goes beyond 3D-CRT by enabling variations of the radiation intensity within each beam.10 New complex computer algorithms provide a means of producing multiple non-cross-firing, coplanar, and noncoplanar beam arrangements that deliver complicated convex dose distributions and steep dose gradients that are ideally suited for parotid neoplasms (Fig. 13–5).
Within individual treatment field angles, multiple beam segments are delivered sequentially to build an intensity map generated by the computer plan that modulates dose around normal adjacent structures.11 For 3D-CRT, optimization of the beam weighting, use of tissue compensators, and determination of beam angles are performed through a series of manual iterations that can be time consuming and require multiple trials if the dose distribution does not meet the physician’s requirements or goals. This approach is called forward planning and is often limited due to the human factor. IMRT employs more sophisticated computer software that can generate IMRT plans through an iterative process called inverse planning, in which beam optimization is performed by the computer rather than manual adjustment. Multiple iterations can be produced and evaluated faster than a manual human approach.10 Inverse computer planning requires the physician to preset dose objectives and constraints prior to beam intensity calculation. The final plan must be accepted by the physician, dosimetrist, and physicist before treatment can begin. Dose volume histograms (DVHs) (Fig. 13–5) are produced that represent graphical illustrations of the IMRT plan and display differential dose distributions to the tumor and normal tissues. Once the IMRT plan is accepted, the data can be automatically transferred to the linear accelerator for future treatment.
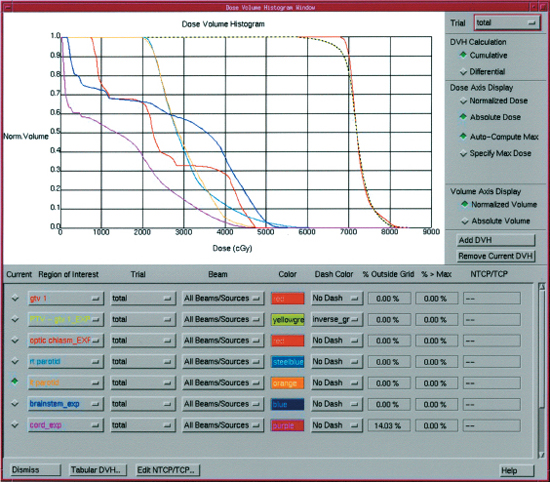
Figure 13-5 Example of a dose volume histogram used to evaluate the dose to the target and normal structures during planning for IMRT.
Treatment, as mentioned earlier, is delivered with automatic blocking devices built in to the head of the machine known as multileaf collimators. The MLCs then form the blocking arrangements in a series of dynamic or static movements to achieve the dose intensity map from the original plan. The decision to use IMRT over 3D-CRT should be made with the input of an IMRT team that includes the physician, physicist, and dosimetrist and should be based on predetermined rationales for IMRT rather than on an ad hoc basis. The design of a departmental treatment algorithm that defines specifically why IMRT would offer advantages in a clinical situation is extremely helpful. The primary reason to employ IMRT for parotid neoplasms is to improve target conformality that delivers an appropriate tumoricidal dose while limiting the dose to adjacent critical structures. A secondary end point is to limit the dose to the contralateral parotid and ipsilateral and/or contralateral submandibular and sublingual glands to preserve remaining salivary function. An example of a situation where IMRT might be beneficial would be in the setting of adenoid cystic cancers where base of skull coverage may be required.
Recent investigations have compared standard 3D-CRT to IMRT to evaluate the role of IMRT for parotid tumors. Bragg et al12 compared IMRT treatment plans with 3D-CRT plans for parotid tumors. For a nine-patient dataset, one 3D-CRT plan was generated for each of nine patients, and 10 IMRT plans with different beam arrangements for each of nine patients. Plans were compared with target dose conformality, dose to organs at risk, and uncomplicated tumor control probability (UTCP). In every case, the IMRT plans produced a higher UTCP than the 3D-CRT plans, suggesting that for a given prescribed dose, the use of IMRT would result in a greater tumor control probability without radiation-induced complications. Target dose was comparable between 3D-CRT and IMRT, but improved when seven to nine IMRT fields were used. IMRT also reduced the mean dose to the contralateral parotid gland, as well as maximum doses to the brain and spinal cord.12 This study was only a comparison of dosimetric outcome looking at dose conformality and critical structure avoidance. Others have reported similar observations regarding improved conformality.13 Thus dose escalation may be feasible with IMRT. If the dose to the target can be increased while maintaining acceptable levels of risk of complications, there should be a resultant improvement in tumor control.12 More beam angles may improve tumor coverage further, but they may also increase overall treatment time. Automated linear accelerators have removed the need for the therapist to enter the treatment suite between fields, making a longer treatment time with a complex IMRT plan acceptable. However, if fewer beam angles or beam segmentations can deliver acceptable target coverage, it should be considered. The planning optimization process that generates an IMRT plan may not always produce a superior plan to 3D-CRT and is not appropriate in every case.
No prospective data have as yet been published to confirm the clinical benefits of IMRT over 3D-CRT in regards to major and minor salivary gland cancers. However, several recent studies have demonstrated the clinical benefit of IMRT in other head and neck sites such as the nasopharynx,14–16 oropharynx, and larynx.17–19
 Future Horizons
Future Horizons
Image-guided Adaptive Radiotherapy
Further advances in linear accelerator design have resulted in the development of hybrid CT accelerators that have the capability to acquire real-time 3D images of the patient for analysis. Setup errors due to patient, organ, or target motion, even up to a couple of millimeters, can be detected and adjusted daily prior to treatment by the physician. As computational algorithms become faster, the potential for adaptive IMRT may be realized. This would allow a daily assessment of tumor response. Reduction in the size of bulky tumors occurs at an average rate of 1.8% per day, but the reduction in tumor volume rarely occurs in a symmetrical manner. It has been observed that parotid glands during a course of radiotherapy tend to contract and shift medially.19 Periodic modifications of the IMRT plan through rapid recalculation of new beam intensity and shape conforming to the changing shape and volume of the tumor and parotid gland may result in a greater therapeutic ratio. Finally, there is a growing desire to incorporate biologic imaging such as PET scans into a four-dimensional model for treatment.
Results of Primary Radiotherapy: Major Salivary Gland Tumors
Results of historical series treating primary or recurrent unresectable disease with external beam alone are presented in Table 13-1 Multiple variables predict for local control, including T stage, histology, dose, fractionation schedule, photons versus neutrons, and recurrent versus unresectable disease, tumor differentiation, and, more recently, the use of chemotherapy.
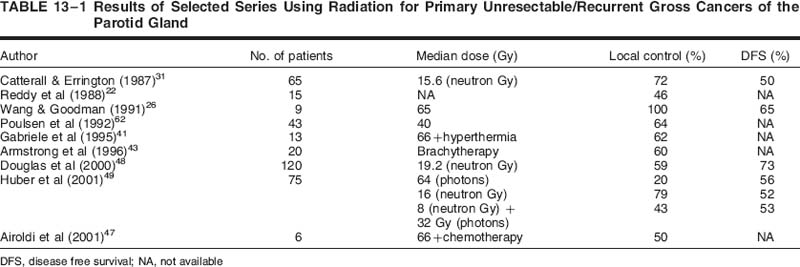
Local control rates for unresectable salivary gland cancers reported with standard fractionated external beam radiotherapy in combination with conventional technology from the 1970s and 1980s were suboptimal, ranging anywhere from 0 to 30%. Treatment results for recurrent tumors are even more dismal with this approach.20–24 Several series retrospectively include together major and minor salivary gland cancers, as well as various histologies and stages. The heterogeneous nature of the cases, radiation doses, and degree of surgery (partial resection vs biopsy) makes it difficult to draw significant conclusions about survival data reported in these earlier studies. This has led to the investigation of alternative approaches to conventional photon radiotherapy in an attempt to improve on prior experiences.
Altered Fractionation
The rationales for altered fractionated photon radiation using conformal delivery techniques include the need to increase dose intensity and/or dose escalation, decrease overall treatment time, and overcome rapid or accelerated repopulation of tumor cells. However, it has been argued that salivary gland tumors are in fact slow growing and rarely display accelerated repopulation, making accelerated hyperfractionation less compelling.25 Wang and Goodman26 reported their results in 1991 using altered fractionation photon irradiation for unresectable parotid and minor salivary tumors. This was the first report in the literature to evaluate this approach for salivary gland cancer. A total of 24 patients with both unresectable major (N = 9) and minor salivary cancers (N = 15) were treated with hyperfractionated photon beam radiotherapy with 1.6 Gy per fraction. Doses ranged from 60 to 78 Gy, with a median dose of 68 Gy. Patients with high-grade lesions received treatment to the ipsilateral neck. Of nine parotid cases, the 5-year actuarial local control at the primary site was 100%, and the survival rate was 65%. Most failures were distant, and there were no reported major complications.26 These results, on the surface, appear impressive. On closer inspection, seven patients (29%) presented with T1- 2N0 tumors, and two patients failed in the neck. If the T1- 2 patients are eliminated, and the two neck failures are included in locoregional control, then the tumor control rates drop to 71%. The median follow-up with elimination of the early-stage patients changes from 43 to 20 months.25 No update or expansion of their salivary gland experience has been published to validate these results with longer follow-up. However, this approach may be a reasonable alternative to high linear energy transfer (LET) radiation, to be discussed later. Hyperfractionation was adopted for all advanced head and neck cancers at the Massachusetts General Hospital and became the investigational arm in a recently completed four-arm randomized trial of altered fractionation versus standard fractionation for advanced head and neck cancers, excluding parotid neoplasms. As a result of this trial, either hyperfractionation (1.2 Gy twice daily) throughout the full course of radiotherapy or concomitant boost (1.5 Gy twice daily weeks 5 and 6) has become the standard of care when chemotherapy is not employed.
At Memorial Sloan-Kettering Cancer Center, all unresectable head and neck cancers were treated beginning in the early 1990s with a delayed concomitant boost (DCB) technique prior to the publication of the Radiation Therapy Oncology Group trial in a prospective phase II trial. Twice-daily treatment was given the last 2 weeks rather than through the entire course. The rationale for the DCB was primarily to overcome the potential accelerated repopulation of tumor cells shown to occur after the fourth week of treatment.27 It also had the advantage of shortening overall treatment from 7 to 6 weeks and intensified the dose. Acute reactions were expected to be greater, but with equivalent late effects similar to conventional fractionation. In a majority of cases, cisplatin chemotherapy was added concurrently on days 1 and 22 of the radiation course.28 Grade 3 mucositis usually occurred during the concomitant boost phase, allowing the majority of patients to complete therapy without requiring a treatment break (76%). This trial included 3 of 82 patients with unresectable parotid tumors, making conclusions about this approach difficult. All three patients with salivary gland neoplasms failed locally.
Results with Charged Particle Irradiation
Charged particles have the potential to improve therapeutic gain based on certain physical and biologic characteristics that differ from photon radiation. Charged particles include neutrons, protons, ions of carbon, helium, and silicon. Protons that have a similar biologic effectiveness relative to photons offer more precise dose localization due to steep dose falloff, whereas high linear energy transfer (LET) particles such as fast neutrons and heavy ions like carbon, helium, and silicon offer a greater relative biologic effectiveness (RBE) to photons. LET is a physical property that is a measure of the mean rate of energy deposited along the track of a charged particle by electromagnetic interactions through a cell matrix. Cell damage is the result of the number of ionizing events produced by the particle track in proximity to the deoxyribonucleic acid (DNA). Charged particles produce either a high or low LET depending on the velocity of the particle traversing the cell. The value of the LET increases as charged particles slow down, resulting in more biologic damage because they cause more severe, less reparable damage per unit of track length than low LET irradiation.29 RBE is a ratio of absorbed doses of two radiations required to produce the same biologic effect. The RBE of a charged particle is dependent on multiple factors, including LET, dose fraction size, tumor hypoxia, and the type of normal tissue adjacent to the tumor. The RBE for protons is thought to be 1.1 to 1.2 relative to photons compared with an RBE of 3.0 for neutrons and 1.2 to 4.5 for helium, neon, silicon, and carbon.29
The oxygen enhancement ratio (OER) is the ratio of dose needed to inactivate hypoxic tumor cells relative to well-oxygenated tumor cells. The degree of tumor hypoxia impacts on tumor repair, and thus the effectiveness of a particular radiation to create DNA damage that is irreparable. The value for protons is 3, which is similar to low LET photons and electrons. The OER is reduced with higher LET radiation-like neutrons, which in turn leads to a higher RBE. Therefore, hypoxic cells normally more resistant to low LET irradiation are more susceptible to cell kill with high LET irradiation (neutrons) because of less dependence on oxygen.
In summary, low LET charged particles derive their advantage from their physical properties, whereas the advantages of high LET radiation include decreased radioresistance of hypoxic tumor cells, decreased repair of radiation cellular damage, and reduced cell cycle dependence.29
Results with Fast Neutrons
Neutron radiation has been investigated at centers in the United States and Europe as an alternative to photon radiation in the treatment of unresectable major and minor salivary gland cancers. It became evident in the 1970s and 1980s that for unresectable advanced or recurrent salivary cancers, conventional photon therapy, although offering improvements in locoregional control, failed to improve overall survival. Recurrent patients did particularly poorly.30 Neutrons, in contrast to photon radiation, deposit energy directly to cellular DNA, as it passes through the cell, and in turn, lead to higher rates of DNA damage. Photons cause cellular damage indirectly, by the creation of free radicals, which in turn cause secondary damage to DNA. The radiobiologic advantages discussed earlier have been put to the test in clinical trials. Catterall and Errington31 published the initial British Medical Research Council (MRC)—Hammersmith Hospital experience in 1986 using fast neutron therapy for locally advanced or recurrent salivary cancers. Treatment was given to 65 patients, 89% of whom were stage IV. Local control and 5-year survival rates were 72% and 50%, respectively. No patients experienced facial nerve functional loss. In fact, in patients with parotid gland tumors, 77% regained or maintained function. The technology to deliver therapy was by today’s standards crude.31 Shortly after this publication, the collective world experience with neutron radiation for major and minor salivary gland cancers was reviewed by Laramore.32 In this retrospective analysis of 309 patients treated, a local control rate of 67% was observed versus 26% for patients treated with photons. Bucholtz et al33 reported a 92% 5-year locoregional tumor control rate in unresectable, previously untreated salivary gland tumors treated with neutrons. This was in comparison to a 5-year local control rate of 51% for recurrent unresectable tumors treated after previous surgery. There was no facial nerve injuries observed in the patients treated with neutrons alone. The authors concluded that the potential morbidity of a debulking surgical procedure before neutron irradiation is not warranted by an improvement in locoregional control over that achievable with neutron therapy alone, and that surgery should be limited to cases with the highest likelihood of achieving negative margins.33
Based on improved outcomes from the MRC and other centers, a prospective randomized trial was performed through the Radiation Therapy Oncology Group and the MRC to confirm the superiority of neutrons. The 10-year local control rate presented in 1993 also favored neutrons over photons (56% vs 17%).34 Krall et al,35 in a review of the European experience, published similar findings in 1998, reporting a local control rate of 65% for neutrons and 28% for photons.35 Prior to this trial, normal tissue toxicity was reported higher with low-energy, fixed beam neutron generators in physics-based laboratories but equivalent to photons when high-energy, hospital-based neutron generators were used.25,31 Normal tissue toxicities in the RTOG—MRC randomized trial between the two groups were not statistically different.
The largest U. S. experience using fast neutrons for unresectable major salivary gland cancers was published al36 in 1999 by Douglas et from the University of Washington. Their experience included 120 patients with either unresectable or gross residual disease after attempted surgery treated with curative intent. It also included 19% with recurrent disease, 39% with gross neck disease, and 11% with prior photon beam therapy. The study included 15% with minor salivary tumors and 32% with adenoid cystic histology. The overall 5-year locoregional control rate and cause-specific survival rates were 58% and 39%, respectively.36 Tumor size was the most important predictor of locoregional control in multivariate analysis. In univariate analysis, surgical debulking appeared to have a superior local control rate (80%) versus 12% if no surgery was attempted. However, this was not evident in multivariate analysis, reflecting the strong influence of tumor size, because tumors ≤4 cm were more likely to undergo some type of surgery. Although tumor size has been reported to be prognostic in other series, the potential for selection bias is evident in this retrospective experience and in others.37 Similarly, primary tumors showed improved locoregional control over recurrent tumors in univariate analysis but not in multivariate analysis. The authors pointed out that this may have been a reflection of tumor bulk. Patients with base of skull involvement and prior radiotherapy also fared poorly. Overall dose and dose constraints to critical structures previously treated limited full curative doses with neutrons that may have accounted for the less favorable outcome in these subsets. Histologic grade was not prognostic for locoregional control, cause-specific survival, or distant metastasis. Histologic subtype, however, was an important prognostic variable. Patients exhibiting adenoid cystic, acinic cell, and basaloid histologies had both superior locoregional control rates and overall survival rates compared with adenocarcinomas. In contrast to other published experiences,38 lymph node involvement alone was not prognostic of locoregional control or survival. However, patients with regional neck metastases were more likely to develop distant metastases (52% vs 32%).
Results with Carbon Ion Radiation
Schulz-Ertner et al39 reported the feasibility of combined photon and carbon ion radiation in 16 patients with locally advanced and residual macroscopic adenoid cystic carcinoma (ACC). The median total tumor dose within the gross tumor volume (GTV) was 72 Gy equivalent. Photon radiation therapy (RT) consisted of fractionated stereotactic RT in seven patients; nine patients received stereotactic intensity-modulated RT. Carbon ion boost was delivered at the heavy ion synchrotron (SIS) at the Heavy Ion Research Center (GSI) in Darmstadt, Germany. With a median follow-up time of 12 months, three patients developed locoregional recurrences 9, 11, and 24 months after RT, respectively. Actuarial local control rates were 80.8% and 64.6% at 1 and 3 years, respectively. Overall survival rates were 100% and 83.3% at 1 and 3 years, respectively. Acute side effects greater than Common Toxicity Criteria (CTC) grade 2 were observed in two patients; no patient developed late effects greater than CTC grade 2.39 Mizoe et al40 reported 5-year local control rates of 90% for unresectable minor salivary ACC and 100% for parotid tumors treated with carbon ion therapy alone in a dose escalation trial in Chiba, Japan. Patients received either 18 fractions through 6 weeks (70 Gy equivalent) or 16 fractions through 4 weeks (64 Gy equivalent) on a phase I/II dose escalation trial. No late grade 3 or 4 toxicity occurred in either arm. Both doses were equivalent in local control.40
The preliminary data from two carbon ion centers have established the safety and efficacy of carbon ion delivery for salivary gland cancers. Overall treatment times are shorter compared with a conventional course of photons, with more favorable dose distributions than neutrons or photons, and the potential for reduced late morbidity. Protons have been used clinically for skull-based tumors, and their role in salivary neoplasms is not established. Certainly their physical dose characteristics make it an attractive alternative to standard 2D and conformal 3D radiation. No meaningful data exist for the use of protons for salivary cancers. However, proton facilities are currently under construction in different regions of the United States for future use in head and neck cancer therapy, as well as other sites of disease.
In summary, the case for high LET radiation therapy is compelling in treating salivary gland tumors, particularly locally advanced, marginally resectable disease and ACC. The differential tissue-sparing effect, cell cycle, and hypoxic insensitivity of high LET, as well as the high RBE combined with more advanced hospital-based systems, make this approach the treatment of choice.
Radiation Combined with Hyperthermia
Local microwave hyperthermia in combination with photon radiation has also been investigated to improve local control over conventional photon irradiation alone. Gabriele et al41 treated 13 patients (20 lesions) with either advanced or recurrent parotid tumors (15 primaries, 5 nodal metastases). Heat was applied twice weekly to a temperature of 428C. Untreated lesions received external beam radiotherapy to 70Gy, and previously treated lesions received 30 Gy. An overall complete response (CR) rate of 80% was observed. The actuarial local control rate at 5 years was 62.3%. No conclusions could be drawn on the impact of tumor size (although the mean maximum diameter for tumors achieving a CR was 3.9 cm compared with 4.25 cm tumors that had a partial response), or on thermal parameters. Acute toxicity was 15% with superficial necrosis. Two of 3 patients healed spontaneously.41 Only one patient suffered a grade 3 late toxicity of fibrosis. Weishedel et al42 published similar results with combined hyperthermia and radiation, obtaining a CR rate of 100% in 16 patients with inoperable ACC at a median follow-up time of 33 months. In comparison with historical patients treated with external beam alone, a 39% improvement in local control was observed (100% vs 61%) and a 20% difference in 5-year survival (57% vs 37%).42 Although crude, the control rates with hyperthermia and photon irradiation appear similar to control rates presented with neutron therapy. Logistically, although the technology of hyperthermia delivery has advanced, not many centers in the United States are still offering hyperthermia as a treatment modality with the exception of a select group of academic settings.
Brachytherapy
Brachytherapy alone or in conjunction with external beam irradiation has been used as a salvage approach in recurrent parotid, submandibular, or sublingual salivary disease after attempted surgical salvage or for unresectable cancers and should be considered if the resources are available, particularly in the setting of prior external beam treatment. Permanent sources such as iodine 125 can be implanted either directly into a tumor or sewn into a Vicryl mesh and placed onto the surface of microscopic or gross residual disease. No dose response data are available, but typically, one aims for a matched peripheral dose of 160 Gy with iodine 125. Likewise, temporary catheters can be implanted in either a planar or multiplanar geometry for the afterloading of iridium 192 sources. General doses range from 45 Gy (microscopic dose) to 60 Gy (gross disease). High dose rate (HDR) fractionated brachytherapy is replacing low dose rate implants because of the reduced exposure to family and hospital personnel. Armstrong et al43 in 20 cases of recurrent or advanced disease reported the use of brachytherapy with iodine 125 or iridium 192. Prior radiation had been delivered in 15 patients. Likewise, 15 patients were implanted with gross residual disease. Despite this, the local control at 5 years was surprisingly good, approaching 60%. Complications included soft tissue necrosis in two patients treated with conservative management and cerebral abscess from skull base exposure treated with surgery and iodine 125, one of which was fatal.43
King and Fletcher44 published a small experience on 16 patients treated with external beam and brachytherapy as a combined initial approach. The local control rate was similar.
Concomitant Chemoradiotherapy
Only a handful of institutions have recently investigated chemotherapy combined with radiotherapy for unresectable or recurrent gross major and minor salivary gland cancers. Airoldi and colleagues45 described six patients with T3–4 inoperable parotid cancers treated with conventional radiotherapy and concurrent cisplatin, 100 mg/m2 on days 1, 22, and 43. Adjuvant cisplatin and etoposide were given for three additional cycles. The median radiation dose was 66 Gy. This regimen achieved a complete response in 3 of 6 patients (50%), partial response in two patients (33%), and stable disease in one patient. The median CR and PR duration was 26 months and 10 months, respectively. Median overall survival was 18 months. No severe late toxicity was observed.45 Agents shown to have moderate activity for parotid and minor salivary cancer include paclitaxel, carboplatin, and vinorelbine and cisplatin.45–47
In summary, although promising response data have been observed, no conclusions can be drawn from the limited data regarding the benefit of concurrent chemoradiotherapy, and it must be considered investigational at this time. However, in certain situations, particularly for unresectable disease in good performance patients, the addition of chemotherapy to radiation therapy is considered acceptable and standard for most advanced head and neck cancers and should be considered if the patient is willing.
Special Management Issues and Outcomes: Unresectable Adenoid Cystic Carcinoma
In many instances, adenoid cystic carcinomas have been included with other unresectable major and minor salivary neoplasms in outcome analyses using conventional radiotherapy. However, several investigators have devoted attention specifically to outcomes related to ACC. As discussed earlier, fast neutron radiation has been investigated as a possible advantage over conventional photons. Douglas et al48 updated the University of Washington experience from 1985 to 1997. One hundred and fifty-nine patients with unresectable, locally advanced or recurrent, nonmetastatic ACC were treated with fast neutron irradiation. Of the total cohort, 29% arose from the parotid gland. The median total dose was 19.2 Gy either given three or four times weekly. For major salivary gland ACC, the 5-year locoregional control, cause-specific survival, and overall survival rates were 67%, 82%, and 71%, respectively. Base of skull invasion, positive lymph nodes, limited biopsy versus attempted surgery, and recurrent tumors were associated with a worse outcome in multivariate analysis. The initial size of the tumor was not significant. Patients presenting for treatment without these negative prognostic factors demonstrated a 5-year cause-specific survival rate of 100%. Lymph node status and base of skull invasion were also associated with a higher rate of distant failure. At 5 years, 50% of patients with regional neck metastases developed distant failure versus 26% of node-negative patients. The high rate of distant failure in the node-negative patients (nearly one third) suggests the presence of micrometastatic deposits and the need to explore better combined systemic approaches. Significant or major complications occurred in ~15% of all patients treated, including minor salivary sites.48
Huber et al49 compared photons and/or electrons to fast neutron therapy for patients with advanced ACC of the head and neck. The 5-year local control rate was 75% for neutrons, versus 32% for photons and for mixed beam (neutrons combined with photons). The improved local control, however, did not translate into a survival advantage. A high rate of distant failure (39%) was observed, with positive lymph nodes associated as the most predictive factor. The absence of positive lymph nodes merely delayed the development of distant failure in a subset of patients, similar to the Washington University experience. Neutrons versus photons, surgery and postoperative radiotherapy versus radiotherapy alone, microscopic versus gross residual or inoperable disease, and smaller tumor size were significantly related to local control in multivariate analysis. Severe toxicity was more prevalent with neutrons (19%) than with photons (4%) or mixed beam (10%). The median dose for patients receiving photons in this review was 64 Gy (range 60–70 Gy). The authors state that patients who underwent surgery received the same dose as patients without surgery, doses by today’s standards considered suboptimal for gross disease and more appropriate for microscopic residual disease. Although in this study major salivary sites represented only 14 of 75 patients (18%), site of origin (major vs minor salivary tissue) did not predict for local control or survival.49
In summary, the data suggest that patients should receive neutron irradiation if possible for unresectable, partially resected, or recurrent unresectable ACC. Local control appears to be superior when compared with conventional photon radiation demonstrating the high RBE of fast neutrons. Cause-specific survival is related to pretreatment tumor bulk or size, histology (ACC vs non-ACC), and the presence of lymph node metastases at presentation. Other factors, such as recurrent disease and base of skull invasion, may again be a reflection of tumor bulk. Due to the high rate of distant failure, primarily a result of lymph node–positive necks, the survival advantage for neutron radiotherapy disappears. The time to distant failure is delayed by a lymph node–negative neck at the time of treatment, as evidenced by the Huber et al49 data (100 vs 16 months), but still occurs to a large degree. The choice of neutrons versus photons must ultimately be made based on individual patient condition and situations. Realistically, fast neutron facilities are not available throughout the United States, necessitating the use of photons either alone or in combination with chemotherapy. A limited number of facilities in the United States and Europe, however, now offer treatment with second-generation cyclotrons using multileaf collimation, which may reduce treatment morbidity through more conformal delivery of neutrons. What needs to be understood is that with the exception of the RTOG—MRC randomized trial, the majority of data are retrospective. Caution should be exercised when interpreting the unresectable data in regards to prognostic variables affecting locoregional control and cause-specific survival.
The unanswered question is if conformal 3D-CRT or IMRT radiotherapy with photons, with doses ≥70 Gy, or dose intensification either with altered fractionation or with concurrent chemotherapy would offer similar outcomes to neutrons with an acceptable level of morbidity. Many of the previously mentioned studies delivered suboptimal photon doses that would be unacceptable by today’s standards. Because local control is an important end point in a disease that has a long survival, despite the high rate of distant failure, prospective trials are needed to address these questions. The possibility, however, of a future trial does not appear likely.
Recurrent Pleomorphic Adenoma
The standard of care for the management of primary pleomorphic adenomas of the parotid gland is superficial or partial superficial parotidectomy with facial nerve preservation, which results in excellent local control with recurrence rates of ~1%. There are situations where the risk of recurrence is higher, and radiotherapy should be considered. The indications for radiotherapy include multiple recurrent lesions, inadequate margins, facial nerve encasement coupled with nerve preserving surgery, gross unresectable disease, gross recurrent disease, and the rare case of malignant transformation. The issues facing a multidisciplinary team include the natural desire on the part of a surgeon to preserve facial nerve function (due to tumor encasement) even in situations that leave microscopic disease behind, coupled with a concern of the potential for second malignancies induced by radiation. These tumors typically occur in young adults. The risk of radiation-induced cancers is small but significant and must be considered. Of equal concern, however, is the potential for multiple surgeries for recurrent disease to increase the risk of deforming facial nerve injury. For subgroups of patients with pleomorphic adenomas encasing the facial nerve, or presenting with microscopic or macroscopic recurrent or multifocal disease, the risk of local failure can be as high as 25 to 50% even after repeat surgery. For high-risk patients, the addition of radiation therapy can reduce local failure rates. These tumors have been shown to be radioresponsive to conventional photons/electrons and neutrons. The typical doses range from 50 to 60 Gy, depending on residual microscopic or macroscopic disease. The risk of regional nodal spread is so low as to not warrant elective nodal irradiation except in the rare case of malignant transformation.
Chon et al50 reviewed the Massachusetts General Hospital experience from 1955 to 1994 of 48 patients treated with surgery and radiation therapy. This study examined the influence of timing of radiotherapy for high-risk patients, comparing a subgroup treated adjuvantly because of high-risk features, with those patients receiving radiotherapy after a first, second, or more recurrence. All patients underwent surgery prior to radiotherapy. The mean follow-up time was 14.5 years. For the 12 high-risk patients treated with immediate adjuvant radiotherapy, the local control rate was 100%. No facial nerve injuries occurred in this group despite doses as high as 70 Gy or with twice-a-day therapy. The local failure rate, however, for first and subsequent recurrences were 7% and 36%, respectively. One patient treated after the third recurrence subsequently developed a squamous cell carcinoma of the buccal mucosa at 15 years. No other malignant transformations occurred. The risk of facial nerve injury rose with more than two surgeries (30%) versus only one (8%). No difference was seen in local control between unifocal and multifocal disease at 10 years.50 Gleave et al51 reported a 5% failure rate in patients with recurrent pleomorphic adenoma treated with surgery and postoperative radiation versus 18% for patients treated with surgery alone. Other authors have published similar local control rates of 90% or better, even in the setting of recurrent disease with conventional postoperative photon/electron radiation.52,53 Buchholz et al54 reported the results of six patients with recurrent disease treated with neutrons. All patients had an average of three prior surgeries. Two patients were treated for gross unresectable disease. With a median follow-up of 52 months, the local control rate was 100%. A low risk of facial nerve injury was observed.54
The risk of radiation-induced carcinogenesis from neutrons is rare and certainly no greater than the risk from conventional photons. The approach to planning, targeting, and delivery of conformal radiotherapy for pleomorphic adenomas is similar to unresectable malignant tumors of the parotid.
Radiation Therapy in the Postoperative Setting: Primary Site
Historical patterns and risk of recurrence have been defined retrospectively by large surgical series. Spiro and associates55 reviewed the patterns of failure of 288 patients after surgery alone for parotid neoplasms. The Memorial Sloan-Kettering group observed an escalating risk of primary failure with increasing stage. The risk of recurrence by stage was 7% stage I, 21% stage II, and 58% stage III.55
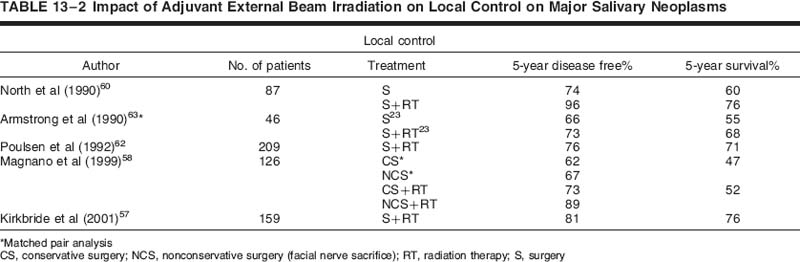
Subsequent to that, Harrison and colleagues56 reviewed the Memorial Sloan-Kettering experience of patients treated with surgery and postoperative conventional radiotherapy for high-risk features. This was not a direct comparison to the earlier Spiro et al55 report, but it provided a sense of improved local control with the addition of radiation. The indications for radiation therapy in this series included positive margins, advanced local disease, positive nodes, and high-grade disease. The overall 5-year actuarial control rates by T stage were 100% for T1, 83% for T2, 80% for T3, and 43% for T4. Patients with positive nodal disease had a lower locoregional control and survival than node-negative patients (58% vs 83%, and 39% vs 80%, respectively). Eight of eight patients (100%) with ACC were locally controlled after adjuvant elective radiation therapy (ERT) in comparison to three of eight (37.5%) treated with surgery alone, with all failures arising within 4 years of surgery. A trend toward improved local control at 10 years was observed with doses of radiation exceeding 57 Gy (72% vs 53%) in comparison with lower doses.56 Other authors have reported similar experiences.57–62 Selected modern series are provided in Table 13-2 showing results with or without adjuvant radiotherapy.
Referral for radiotherapy is based many times on surgical bias and is typically a patient presenting after surgery with poor prognostic features, as discussed previously. Further complicating any meaningful conclusions of the efficacy of radiotherapy is the heterogeneity of surgical technique (conservative vs radical parotidectomy) and radiation dose and technique. Armstrong et al63 performed a match-paired analysis of surgical patients to balance out pretreatment variables to determine the efficacy of adjuvant radiation. This analysis represents the closest thing to a prospective evenly matched randomized trial in the literature. In this unique study, 46 patients treated with surgery and postoperative radiation were compared with 46 patients treated with surgery alone. A large group of surgical patients treated at Memorial Sloan-Kettering Cancer Center allowed Armstrong to pick out at least 46 patients for well-matched pairs. The analysis showed that surgery alone was adequate for stage I and II salivary cancers. For T3 and T4 tumors and tumors with positive nodal disease, a significant improvement in locoregional control was observed with postoperative radiation. The 5-year locoregional control and determinate survival rates for stage I/II comparing surgery versus surgery and radiation was 91% versus 79%, and 96% versus 82%, respectively. For stage III/IV disease, the 5-year loco-regional control and determinate survival rates comparing surgery and surgery plus radiation were 17% versus 51%, and 10% versus 51%, respectively. As one can see, despite the improved local control with a combined approach, the failure rate even with the addition of radiotherapy was suboptimal, particularly for T4 tumors. A trend toward improved survival was also seen for high-grade cancers. Although the indications for radiotherapy seem reasonably clear based on the collective experiences, it is vital that a multidisciplinary approach toward the individual patient be performed with all subspecialties in head and neck cancer management giving input to consider all nuances and recommend a comprehensive strategy.
Postoperative Management of Adenoid Cystic Cancers
The reason ACC is addressed separately is the unique behavior of these tumors, combined with the specific issue of how to adequately address close/positive margins and/or perineural spread as it relates to radiotherapy coverage of the base of the skull and total dose. For stage I tumors with negative margins and no perineural spread, there is no need for adjuvant radiotherapy.64 For more advanced disease, several authors have shown improved local control with the addition of adjuvant radiation to surgery for ACC, ranging from 72 to 86% with and 11 to 47% without.65–67 Garden and colleagues68 addressed the above concerns in a published retrospective analysis of a 30-year experience from the M. D. Anderson Cancer Center. This study was unique in its comprehensive policy of offering a multimodality approach to ACC. Between 1962 and 1991, 198 patients received postoperative radiotherapy after surgery for suspected or known microscopic positive margins. Of the total, 71 patients had major salivary ACC, and 122 presented with minor salivary ACC. Over two thirds (69%) of the entire cohort had perineural spread. The median radiation dose to the tumor bed and follow-up time was 60 Gy and 93 months. respectively. The 10 and 15 actuarial local control rates were 86% and 79%, respectively. Positive margins had a significant impact on local failure compared with close or negative margins. The failure rates were 18%, 9%, and 5%, respectively. Perineural invasion was significant only if a major nerve was involved. Patients with perineural spread of a major named nerve demonstrated crude local failure rates of 18% compared with 9% without. The dose of radiotherapy had a dramatic impact on local control when positive margins were present. Crude control rates were 40% and 88% for doses <56 Gy and ≥56 Gy. The 10year local control rate declined from 93% with negative margins and no perineural spread, to 83% with either perineural invasion or positive margins, and to 70% when both were present. The site of disease was not a factor in local control. Four patients (2%) failed at the base of the skull, three of four presenting with major nerve involvement. The distant failure rate was 37%, despite local control in 31%. Distant failure rates were no different for negative versus positive margins, but they were higher when stratified by named nerve involvement. The authors recommended a dose of 57 to 60 Gy to the operative bed for negative or close margins and 66 Gy for positive margins. The neck was treated only in cases of positive nodes. Neck failure was rare.
In summary, patients with ACC should receive adjuvant radiotherapy if close or positive margins are suspected or if perineural invasion is evident. Elective coverage of the base of the skull should be included in a radiation port if a major nerve is involved or if the margins of an involved nerve at the proximal end are positive as it is dissected toward the skull base. Focal perineural invasion is not an indication to electively treat the base of the skull. In some cases, base of the skull inclusion is unavoidable by virtue of the location of the nerve pathway in relation to the primary site, such as the parotid or paranasal sinus regions.68 Routine involvement of the base of the skull for a sublingual or submandibular site would result in excessive morbidity. Dose is critical and should be tailored based on presentation of margins and nerve involvement. Finally, the high rate of distant failure requires a serious look at adding chemotherapy to the comprehensive management of ACC in a prospective trial.
Elective Treatment of the Neck
Uniform elective treatment of the neck is not indicated after surgical resection of the primary site. The largest study to specifically look at risk variables that predict for microscopic neck metastasis was performed by Armstrong et al2 in 1992. This analysis was designed to outline indications for elective neck radiation (ENR). The records of 474 patients who underwent surgical resection of a locally confined major salivary gland cancer at Memorial Sloan-Kettering Cancer Center were reviewed. Clinically positive nodes presented in 14%, and clinically occult, pathologically positive nodes occurred in 12% of the 474 patients. In multivariate analysis, tumor size and grade were significant risk factors. Tumors 4 cm or greater had a 20% risk of occult nodal metastases compared with 4% risk for tumors less than 4 cm. High-grade tumors regardless of histologic type had a 49% risk compared with a 7% risk for intermediate-low-risk tumors. What should the radiation oncologist do in the setting of positive occult nodal disease after elective nodal dissection (END)? Armstrong and colleagues2 also addressed this. The failure rate in patients who underwent END and were found to have occult disease and did not receive radiotherapy was 29%, compared with the 0% failure rate in patients who did receive radiotherapy. Another point brought out by the authors is the high rate of occult neck disease with epidermoid carcinomas, and the concern that the primary site may have been cutaneous in origin. What is not addressed in this or any study is the relative efficacy of ENR versus END in controlling occult disease. It is fair to extrapolate for epidermoid cancers of other head and neck sites that ENR in doses between 45 and 50 Gy will control occult microscopic disease in the neck in greater than 95% of patients. More interestingly, selective ENR can be performed similar to selective END. In this series, a parotid and level II neck dissection would have missed occult disease in 25% of cases. The addition of level III nodes would have reduced this to 10%.
Radiation Target Coverage
For tumors of parotid origin, it would appear reasonable to include the parotid bed up to the base of the skull for deep lobe invasion, along with nodal levels II, III, and IV with exclusion of levels Ia, Ib, Va, and Vb. For submandibular or sublingual origin, levels Ia, Ib, II a, IIb, III, and IV would appear appropriate with exclusion of levels Va and Vb. This would reduce morbidity associated with ENR. For parotid and submandibular sites, there is no need to treat the contralateral neck, even in the setting of gross nodal disease of the ipsilateral side. For sublingual origin, the risk of contralateral spread increases due to the proximity to the midline. This necessitates inclusion of the contralateral level Ia–b, IIa–b, and III nodal regions. Fortunately, the parotid glands can be spared from this approach, as the likelihood of occult disease is low.
Primary Radiotherapy of Minor Salivary Gland Cancers
The indications for choosing primary radiotherapy over surgery are based on site accessibility, extent of local infiltration, resectability, and potential functional/cosmetic loss and medical operability. Because minor salivary glandular tissue arises in multiple sites throughout the oral cavity, oropharynx, nasal and paranasal cavities, larynx, and hypopharynx treatment must be individualized. For example, minor salivary gland cancers are more common on the hard and soft palate than the larynx or hypopharynx. The functional loss of the hard palate and/or soft palate for some patients is devastating, and primary radiotherapy is a reasonable alternative. The risk of neck disease is also low, allowing for more conformal radiation techniques like IMRT to the primary site. Likewise, surgery for the nasopharynx is rarely performed. Minor salivary gland cancers arising in the nasal and paranasal sinuses are usually quite extensive at presentation. Many are unresectable, or would require morbid surgery, depending on the extent of spread to critical surrounding structures.
Radiation doses are similar to doses prescribed for major salivary tumors (66–70 Gy). Elective treatment of the neck is reserved for high-grade lesions and site of origin, rather than histology. Minor salivary gland cancers of the lip, buccal mucosa, palate, and sinonasal tract rarely metastasize regionally and do not require ENR or END.69 Intermediate-risk sites (oral tongue, floor of the mouth), and high-risk sites (nasopharynx, oropharynx, larynx, and hypopharynx) should be considered for ENR. Doses for ENR are similar to that for major salivary gland cancers.
Results of Primary Radiotherapy: Minor Salivary Gland
Parsons et al69 published the largest experience with primary radiotherapy in the United States. The outcomes of 95 patients treated at the University of Florida were reviewed, 45 of whom received radiotherapy alone. The predominant site of presentation was the oral cavity, followed by nasal/paranasal sinuses and oropharynx. Overall, local control for patients treated with radiotherapy alone was 21 of 45, ranging from 2.5 to 21 years, including 12 patients with ACC. Local control results according to tumor site showed 64% for oral cavity, 83% for oropharynx, 22% for nasal/paranasal sinuses and nasopharynx (presented together), and 100% for larynx and hypopharynx. Doses of 65 Gy produced a high rate of local control, whereas higher doses (70 Gy) resulted in even higher rates of local control for advanced disease. This was even more evident for adenoid cystic cancers, with local control rates of 2 of 12 for < 70 Gy compared with 10 of 13 for doses ≥ 70 Gy. There was no significant difference in local control based on histologic type. Higher failure rates within the nasal/ paranasal group were more likely a result of extent of disease rather than site. In multivariate analysis of factors influencing local control, combined surgery and radiation was superior to radiotherapy alone, along with T stage.69 Despite improved local control rates with combined treatment, the authors noted that the 10-year absolute and cause-specific survival rates with combined treatment were similar to surgery alone results reported by Spiro et al71 from Memorial Sloan-Kettering Cancer Center. The 10-year cause-specific survival rates at Memorial Sloan-Kettering were 93% stage I, 68% stage II, 58% stage III, and 24% stage IV, respectively, compared with 75%, 58%, and 27% for stage I–II, stage III, and stage IV, respectively, from Parsons et al.69 Neck failure occurred in only 8% of 39 patients not electively treated. Perineural invasion was not addressed in this study, but Garden et al68 observed a higher rate of base of skull and distant failure with major named nerve involvement.
Results and Factors Affecting Outcome of Combined Surgery and Radiation Therapy for Minor Salivary Gland Cancer
Postoperative radiotherapy is not indicated for low-grade, early-stage lesions when negative margins are obtained. The indications for radiotherapy in the postoperative setting are similar to major salivary cancers and include positive or close margins, perineural spread, vascular space invasion, T3–4 tumors or tumor with penetration into underlying bone, skeletal muscle, or cartilage, high-grade tumors, multiple nodal involvement, and recurrent disease. Factors affecting local control and survival are not always clear based on conflicting data.69,70 The local failure rates for 160 patients treated with combined surgery and radiotherapy in the M. D. Anderson Cancer Center experience was 12%.68 In contrast, a local failure rate of 48% in patients treated with surgery alone was reported from the Memorial Sloan-Kettering Center.71 Sadeghi and colleagues72 also showed higher local control rates with the addition of radiotherapy in 47 patients with positive margins after surgery. Local control was 76% in the group of patients receiving radiotherapy versus 47% for those who did not.72 Parsons et al69 reported 10-year local control rates with combined treatment of 100% for T1–2 tumors, 100% for T3 tumors, and 33% for T4 tumors. Spiro et al73 found overall stage to be the most important predictive factor for survival in 378 patients retrospectively staged using the AJCC criteria available at that time. Parsons and associates69 also observed overall stage to be the most significant factor in survival. The site of origin as an independent prognostic variable in determining outcome is also not clear. Reports of lower local control and survival for nasal cavity and paranasal sinus tumors, for example, are probably a reflection of T stage and not the site of disease.69 Most investigators report an influence of tumor grade rather than histology on survival.69–71
Adenoid cystic carcinomas arising in minor salivary sites, similar to major salivary tumors, have a high propensity for distant spread, approaching 40% in some studies.69 Despite this, the indolent nature of ACC in many cases results in 5-year survival rates approaching 40%.67,69 Perineural spread has been observed in nearly 60% of minor salivary gland cancers after surgery.70 The base of the skull should be irradiated only if a major nerve branch is involved with tumor.68 Based on regional neck failure patterns, radiotherapy to the involved neck should be considered if multiple lymph nodes harbor disease or if extracapsular penetration is found.68 It is important to note that a single metastatic lymph node after a selective neck dissection means the neck is still at risk for failure and should be treated with radiotherapy. Although the lymph node drainage patterns by site are generally orderly, midline cancers and tumors arising in sites with rich lymphatic drainage such as nasopharynx, base of the tongue, and larynx should have both necks addressed electively with either surgery or radiotherapy.
Radioprotectors
Chemical radioprotector compounds were initially investigated to provide protection against whole-body radiation exposure in the event of a nuclear catastrophe. The Walter Reed Army Research Institute developed a thiophosphate, WR-2721, as part of a vast development program during the cold war to provide radiation protection to soldiers. WR-2721, also known as amifostine, has been investigated as a radioprotector in oncology to improve the therapeutic ratio, based on the assumption that a differential uptake of the compound would occur, with higher concentrations in normal tissue compared with tumor cells, leading to greater normal tissue protection.74 Amifostine requires a dephosphorylation of its phosphate group by alkaline phosphatase to convert into the more active free thiol WR-1065. Normal tissues contain higher concentrations of this enzyme, leading to greater uptake of WR-2721 in normal cells. The active form, WR-1065, acts intracellularly to scavenge and bind oxygen-free radicals and assist in DNA repair after radiation exposure.75 There also appears to be some degree of chemoprotection with differential sparing of bone marrow and intestinal mucosa with cisplatin, an active agent used in head and neck cancer, as well as alkylating agents.76,77 Human clinical trials have subsequently been performed in bone marrow cancer, lung cancer, cervical cancer, rectal cancer, and head and neck cancer. Amifostine can be administered either intravenously as a rapid push or subcutaneously 30 to 45 minutes prior to radiation. A phase III randomized trial in head and neck cancer using radiation with or without amifostine administered intravenously was performed with the end point of xerostomia and unstimulated salivary flow. The incidence of grade 2 xerostomia was reduced from 78 to 51%. No tumor protection was observed. Side effects included nausea, vomiting, hypotension, and anorexia.78 Subsequent investigations are under way to determine the efficacy of amifostine delivered subcutaneously compared with intravenous delivery. It is thought that subcutaneous delivery reduces the degree of nausea and hypotension, making the addition to radiotherapy or chemoradiotherapy more tolerable. Robust oral hydration is essential both before and after administration to reduce nausea and hypotension. Recently published data have dispelled the concern of any tumor protective effect from amifostine.78,79 The implications for major and minor salivary gland protection are compelling, particularly sparing of the contralateral submandibular gland and parotid from cross-firing and exit beam dose. Amifostine is now being used for head and neck cancer in the United States and elsewhere to reduce xerostomia and acute and late toxicity associated with combined chemoradiotherapy.
Radiation Morbidity
Critical Structures
The proximity of the parotid and other salivary glandular tissue to several critical normal structures in the head and neck makes the task of delivering tumoricidal doses of radiation a challenge. These structures include the mandible, spinal cord, brainstem, temporal lobe, eye, and inner ear. Exceeding the tolerance of these tissues can result in severe late toxicity. Late toxicity would include mandibular necrosis, spinal cord injury resulting in paresis or paralysis, brainstem injury leading to respiratory failure and/or paralysis, temporal lobe necrosis, blindness, and loss of hearing. The dose-limiting tolerance of these tissues has been documented over the years from a compilation of clinical and radiobiologic studies and should be heeded. The threshold doses for various tissue or organs have been defined as the TD5/5, or threshold dose expected to causes a 5% complication at 5 years.8 However, in select cases, a decision must be made to exceed the TD 5/5 to obtain reasonable target coverage. This should be discussed with the patient and documented in writing. Pre-radiation dental evaluation is essential and advised for all patients. Mandibular complications can be reduced with preventive dental evaluation and extraction of ipsilateral teeth that are distressed. Failure to do so can lead to future infection of the bone. Other dental maneuvers to reduce acute reactions during treatment include the replacement of metallic fillings with composites to reduce radiation scatter to the tongue and the fashioning of a lead-lined dental mold placed intraorally through treatment to reduce scatter.
Less Critical Structures
Less critical but important structures include the salivary glands, temporomandibular joint, middle ear, facial and scalp hair, thyroid gland, oral and nasal mucosa, and lacrimal gland. Late side effects to these structures are not life threatening but can contribute to a reduction in functional quality of life. IMRT, 3D-CRT, or protons can be used to spare ipsilateral or contralateral salivary tissue to reduce the risk of xerostomia. The risk of late xerostomia is related to the mean dose to either the parotid or submandibular gland. As the mean dose rises above 27 Gy, the chance of significant recovery decreases.7
Acute toxicity generally occurs 3 to 4 weeks into therapy and is related to multiple factors, including diabetes, Sjögren’s syndrome, dose per fraction, accelerated fractionation, concurrent chemotherapy, prior surgery, prior radiotherapy, and the use of amifostine. Acute effects include xerostomia, dental caries, epilation of facial and scalp hair, skin erythema, changes to or loss of taste, oral candidiasis, progressive mucositis of the oral cavity and oropharynx, esophagitis, and acute sialadenitis.
The occurrence and severity of a late toxicity are dependent on factors such as the location and volume of normal tissue treated, total dose and dose per fraction, conformality of the radiation beam arrangements, chemotherapy, lack of proper dental attention prior to therapy, prior surgery or radiotherapy, recurrent disease, and the use of radioprotectors.
Late side effects include the risk of trismus if a large portion of the temporomandibular joint and/or masseter muscle is exposed to doses greater than 50 Gy, otitis media when the entrance or exit beam is through the middle ear, alopecia due to exit dose, skin and dermal fibrosis that can be exacerbated by prior therapies, chemical, subclinical, or clinical hypothyroidism, and mandibular osteoradionecrosis.
Several studies have shown promise with early intervention of different agents to reduce and reverse acute as well as late toxicity. Antifungal agents, nonsteroidal anti-inflammatory agents, zinc, and pentoxifylline are some of the compounds that have been investigated that appear to reduce some of the acute and late toxicity by limiting mucositis and xerostomia, as well as soft tissue and muscular fibrosis.80–83 Further clinical development of pharmacological approaches to modification of chronic radiation injuries could lead to significant improvement in the quality of life for radiotherapy head and neck patients
REFERENCES
1. Spiro, RH Armstrong, J Harrison, L Geller, NL Lin, SY Strong, EW. Carcinoma of major salivary glands: recent trends. Arch Otolaryngol Head Neck Surg 1989; 115: 316–321
2. Armstrong, JG Harrison, LB Thaler, HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer 1992; 69: 615–619
3. Nowak, PJ Wijers, OD Lagerwaard, FJ Levendag, PC. A threedimensional CT-based target definition for elective irradiation of the neck. Int J Radiat Oncol Biol Phys 1999; 45 (1): 33–39
4. Wijers, OB Levendag, PC Tan, T, et al. A simplified CT-based definition of the lymph node levels in the node negative neck. Radiother Oncol 1999; 52: 35–42
5. Chao, CKS Wippold, FJ Ozyigit, G Tran, B Dempsey, JF. Determination and delineation of nodal target volumes for head and neck cancer based on patterns of failure in patients receiving definitive and postoperative IMRT. Int J Radiat Oncol Biol Phys 2002; 55 (5): 1174–1184
6. Gregoire, O Scalliot, P Ang, KK. Clinical target volumes in conformal and intensity modulated radiation therapy. Berlin: Springer Science + Business Media; 2004
7. Eisbruch, A Ten Haken, RK Kim, HM, et al. Dose, volume and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 45: 577–587
8. Emami, B Lyman, J Brown, A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–122
9. Losasso, T Chui, CS Kutcher, GJ, et al. The use of a multi-leaf collimator for conformal radiotherapy of carcinoma of the prostate and nasopharynx. Int J Radiat Oncol Biol Phys 1993; 25: 161–170
10. Xia, P Amols, HI Ling, C. Three-dimensional conformal radiotherapy and intensity-modulated radiotherapy. In: Leibel, S Phillips, T, eds. Textbook of Radiation Oncology, 2nd ed. Philadelphia: WBSaunders; 2004: 163
11. Meeks, SL Buatti, JM Bova, FJ, et al. Potential clinical efficacy of intensity-modulated radiation conformal therapy. Int J Radiat Oncol Biol Phys 1998; 40: 483–495
12. Bragg, CM Conway, J Robinson, MH. The role of intensitymodulated radiotherapy in the treatment of parotid tumors. Int J Radiat Oncol Biol Phys 2002; 52 (3): 729–738
13. Nutting, CM Rowbottom, CG Cosgrove, VP, et al. Optimisation of radiotherapy for carcinoma of the parotid gland: a comparison of conventional, three-dimensional conformal, and intensitymodulated techniques. Radiother Oncol 2001; 60: 163–172
14. Lee, N Xia, P Fischbein, NJ Akazawa, C Quivey, JM. Intensitymodulated radiation therapy for head and neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys 2003; 57 (1): 49–60
15. Hunt, MA Zelefsky, MJ Wolden, S, et al. Treatment planning and delivery of intensity-modulated radiation therapy for nasopharynx cancer. Int J Radiat Oncol Biol Phys 2001; 49 (3): 623–632
16. Xia, P Fu, KK Wong, GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000; 48: 329–337
17. Chao, KS Ozyigit, G Tran, BN Cengiz, M Dempsey, JF Low, DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head and neck cancer. Int J Radiat Oncol Biol Phys 2003; 55 (2): 312–321
18. Chao, KS Ozyigit, G Blanco, AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma:impact of tumor volume. Int J Radiat Oncol Biol Phys 2004; 59 (1): 43–50
19. Garden, AS Morrison, WH Rosenthal, DI Chao, KS Ang, KK. Target coverage for head and neck cancers treated with IMRT: review of clinical experiences. Semin Radiat Oncol 2004; 14 (2): 103–109
20. Rafla, S. Malignant parotid tumors: natural history and treatment. Cancer 1977; 40: 136–144
21. Gabriele, P Orecchia, R Boidi-Trotti, A Orecchia, A Ragona, R. La radioterapia nel trattamento dei carcinomi delle ghiandole salivari maggiori. Radiol Med (Torino) 1988; 76: 316–322
22. Reddy, SP Hines, IL Marks, JE. Treatment of locally advanced, high grade, malignant tumors of major salivary glands. Laryngoscope 1988; 98: 450–454
23. Laramore, GE. Fast neutron radiotherapy for inoperable salivary gland tumors: is it the treatment of choice? Int J Radiat Oncol Biol Phys 1987; 13: 1421
24. Fu, KK Leibel, S Levine, ML, et al. Carcinoma of the major and minor salivary glands: analysis of treatment results and sites and causes of failures. Cancer 1977; 40: 2882–2890
25. Griffin, TW. Optimal treatment for salivary gland tumors. Int J Radiat Oncol Biol Phys 1991; 21: 857–858
26. Wang, CC Goodman, M. Photon irradiation of unresectable carcinomas of the salivary glands. Int J Radiat Oncol Biol Phys 1991; 21 (3): 569–576
27. Taylor, JM Withers, HR Mendenhall, WM. Dose-time considerations of head and neck squamous cell carcinomas treated with irradiation. Radiother Oncol 1990; 17 (2): 95–102
28. Harrison, LB Raben, A Pfister, D, et al. A prospective phase II trial of concomitant chemotherapy and radiotherapy with delayed accelerated fractionation in unresectable tumors of the head and neck. Head Neck 1998; 20 (6): 497–503
29. Castro, JP Petti, PL Blakely, EA Daftari, IK. Particle radiation therapy. In: Leibel, Sa Phillips, TL, eds. Textbook of radiation oncology, 2nd ed. Philadelphia: WB Saunders; 2004: 1548, 1549
30. Armstrong, JG Harrison, LB Spiro, RH, et al. Observations on the natural history and treatment of recurrent major salivary gland cancer. J Surg Oncol 1990; 44: 138–141
31. Catterall, M Errington, RD. The implications of improved treatment of malignant salivary gland tumors by fast neutron radiotherapy. Int J Radiat Oncol Biol Phys 1987; 13: 1313–1318
32. Laramore, GE. Fast neutron radiotherapy for inoperable salivary gland tumors: is it the treatment of choice? Int J Radiat Oncol Biol Phys 1987; 13: 1421–1423
33. Buchholz, TA Laramore, GE Griffin, BR Koh, WJ Griffin, TW. The role of fast neutron radiation therapy in the management of advanced salivary gland malignant neoplasms. Cancer 1992; 69 (11): 2779–2788
34. Laramore, GE Krall, JM Griffen, TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial. Int J Radiat Oncol Biol Phys 1993; 27: 235–240
35. Krull, A Schwarz, R Brackrock, S, et al. Neutron therapy in malignant salivary tumors: results at European centers. Recent Results Cancer Res 1998; 150: 88–99
36. Douglas, JG Laramore, GE Austin-Seymour, M, et al. Neutron radiotherapy for the treatment of locally advanced salivary gland cancers. Head Neck 1999; 21 (3): 255–263
37. Spiro, RH. Changing trends in the management of salivary tumors. Semin Surg Oncol 1995; 11: 240–245
38. Rodriguez-Cuevas, S Labastida, S Baena, L. Risk of nodal metastases from malignant salivary gland tumors related to tumor size and grade of malignancy. Eur Arch Otorhinolaryngol 1995; 252: 139–142
39. Schulz-Ertner, D Nikoghosyan, A Jakel, O, et al. Feasibility and toxicity of combined photon and carbon ion radiotherapy for locally advanced adenoid cystic carcinomas. Int J Radiat Oncol Biol Phys 2003; 56 (2): 391–398
40. Mizoe, JE Tsujii, H Kamada, T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004; 60 (2): 358–364
41. Gabriele, P Amichetti, M Orecchia, R Valdagni, R. Hyperthermia and radiation therapy for inoperable or recurrent parotid carcinoma: a phase I/II study. Cancer 1995; 75: 908–913
42. Weishedel, U Wieland, C. Results in combined hyperthermia/ radiotherapy of adenoid cystic carcinomas compared with conventional treatment. Strahlenther Onkol 1987; 163: 714–717
43. Armstrong, JG Harrison, LB Spiro, RH, et al. Brachytherapy of malignant tumors of salivary origin. Endocurither Hypertherm Oncol 1996; 6: 19
44. King, JJ Fletcher, GH. Malignant tumors of the major salivary glands. Radiology 1971; 100: 381–384
45. Airoldi, M Gabriele, P Pedani, F, et al. Concomitant chemoradiotherapy followed by adjuvant chemotherapy in parotid gland undifferentiated carcinoma. Tumori 2001; 87 (1): 14–17
46. Airoldi, M Fornari, G Pedani, F, et al. Paclitaxel and carboplatin for recurrent salivary gland malignancies. Anticancer Res 2000; 20: 3781–3783
47. Airoldi, M Pedani, F Succo, G, et al. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer 2001; 91: 541–554
48. Douglas, JG Laramore, GE Austin-Seymour, M, et al. Treatment of locally advanced adenoid cystic carcinoma of the head and neck with neutron radiotherapy. Int J Radiat Oncol Biol Phys 2000; 46 (3): 551–557
49. Huber, PE Debus, J Zierhut, D Bischof, M Wannenmacher, M Engenhart-Cabillic, R. Radiotherapy for advanced adenoid cystic carcinoma: neutrons, photons or mixed beam? Radiother Oncol 2001; 59: 161–167
50. Chon, B Caubet, JF Wang, CC. Optimum management of pleomorphic adenoma of the parotid gland. Int J Radiat Oncol Biol Phys 2000; 48 (3): 266, 267
51. Gleave, EN Whittaker, JS Nicholson, A. Salivary tumors: experience over thirty years. Clin Otolaryngol Allied Sci 1979; 4: 247–257
52. Dawson, AK. Radiation therapy in recurrent pleomorphic adenoma of the parotid. Int J Radiat Oncol Biol Phys 1989; 16: 819–821
53. Ravasz, LA Terhaard, CH Hordiijk, GJ. Radiotherapy in epithelial tumors of the parotid gland: case presentation and literature review. Int J Radiat Oncol Biol Phys 1990; 19 (1): 55–59
54. Buchholz, TA Laramore, GE Griffin, TW. Fast neutron radiation for recurrent pleomorphic adenomas of the parotid gland. Am J Clin Oncol 1992; 15 (5): 441–445
55. Spiro, RH Huvos, AG Strong, EW. Cancer of the parotid gland: a clinicopathologic study of 288 primary cases. Am J Surg 1975; 130: 452–459
56. Harrison, LB Armstrong, JG Spiro, RH, et al. Postoperative radiation therapy for major salivary gland malignancies. J Surg Oncol 1990; 45: 52–55
57. Kirkbride, P Liu, FF O’Sullivan, B, et al. Outcome of curative management of the parotid gland. J Otolaryngol. 2001; 30 (5): 271–279
58. Magnano, M Gervasio, F Cravero, L, et al. Treatment of malignant neoplasms of the parotid gland. Otolaryngol Head Neck Surg 1999; 121: 627–632
59. Spiro, IJ Wang, CC Montgomery, WM. Carcinoma of the parotid gland: analysis of treatment results of surgery and patterns of failure after combined surgery and radiation therapy. Cancer 1993; 71: 2699–2705
60. North, CA Lee, DJ Piantadosi, S, et al. Carcinoma of the major salivary glands treated by surgery or surgery plus postoperative radiotherapy. Int J Radiat Oncol Biol Phys 1990; 18: 1319–1326
61. Eapen, LJ Gerig, LH Catton, GE, et al. Impact of local radiation in the management of salivary gland carcinomaa. Head Neck Surg 1988; 10: 239–245
62. Poulsen, MG Pratt, GR Kynaston, B Tripcony, LB. Prognostic variables in malignant epithelial tumors of the parotid. Int J Radiat Oncol Biol Phys 1992; 23: 327–332
63. Armstrong, JG Harrison, LB Spiro, RH, et al. Malignant tumors of major salivary gland origin: a matched-pair analysis of the role of combined surgery and radiotherapy. Arch Otolaryngol Head Neck Surg 1990; 116: 290–293
64. Spiro, RH Huvos, AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg 1992; 164: 623–628
65. Simpson, J Thawley, S Matsuba, H. Adenoid cystic salivary gland carcinoma: treatment with irradiation and surgery. Radiology 1984; 151: 509–512
66. Black, KM Fitzpatrick, PJ Palmer, JA. Adenoid cystic carcinoma of the salivary glands. Can J Surg 1980; 23: 32–35
67. Miglianico, L Eschwege, F Marandas, P, et al. Cervico-facial adenoid cystic carcinoma: study of 102 cases. Influence of radiation therapy. Int J Radiat Oncol Biol Phys 1987; 13: 673–678
68. Garden, AS Weber, RS Morrison, WH Ang, KK Peters, LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995; 32 (3): 619–626
69. Parsons, JT Mendenhall, WM Stringer, SP, et al. Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys 1996; 35 (3): 443–454
70. Weber, RS Palmer, M El-Naggar, A, et al. Minor salivary gland tumors of the lip and buccal mucosa. Laryngoscope 1989; 99: 6–9
71. Spiro, RH Koss, LG Hajdu, SI, et al. Tumors of minor salivary gland origin—a clinicopathologic study of 492 cases. Cancer 1973; 31: 117–129
72. Sadeghi, A Tran, LM Mark, R Sidrys, J Parker, RG. Minor salivary gland tumors of the head and neck: treatment strategies and prognosis. Am J Clin Oncol 1993; 16 (1): 3–8
73. Spiro, RH Thaler, HT Hicks, WF, et al. The importance of clinical staging of minor salivary gland carcinoma. Am J Surg 1991; 162: 330–336
74. Denekamp, J Rojas, A Stewart, FA. Is radioprotection by WR-2721 restricted to normal tissues? In: Nygaard, OF Smic, MG, eds. Radioprotectors and anticarcinogens. New York: Academic Press; 1983: 655
75. Urtasun, RC Brown, MJ. Chemical modifiers of radiation. In: Leibel, Sa Phillips, TL, eds. Textbook of radiation oncology, 2nd ed. Philadelphia: WB Saunders; 2004: 55
76. Wasserman, TH Phillips, TL Ross, G Kane, LJ. Differential protection against cytotoxic chemotherapeutic effects on bone marrow CFUs by Wr-2721. Cancer Clin Trials 1981; 4: 3–6
77. Peters, GJ van der Wilt, CL Gyergyay, F, et al. Protection by WR- 2721 of the toxicity induced by the combination of cisplatin and 5- fluorouracil. Int J Radiat Oncol Biol Phys 1992; 22: 785–789
78. Brizel, DM Wasserman, TH Strand, V, et al. Final report of phase III randomized trial of amifostine as a radioprotectant in head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 45: 147
79. Komaki, R Lee, JS Milas, L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys 2004; 1;58 (5): 1369–1377
80. Dijkstra, PU Kalk, WW Roodenburg, JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004; 40 (9): 879–889
81. Ledee-Bataille, N Olivennes, F Lefaix, JL, et al. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Hum Reprod 2002; 17 (5): 1249–1253
82. Delanian, S Balla-Mekias, S Lefaix, JL, et al. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999; 17 (10): 3283–3290
83. Ertekin, MV Koc, M Karslioglu, I Sezen, O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys 2004; 58 (1): 167–174
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


