CHAPTER 9 THE IMPACT OF TRANSMISSIBLE DISEASE ON THE PRACTICE OF DENTISTRY
ACQUIRED IMMUNODEFICIENCY SYNDROME
No single factor has affected the practice of dentistry since the early 1980s more than acquired immunodeficiency syndrome (AIDS). “Once upon a time, no one in the world had ever heard of the acquired immunodeficiency syndrome (AIDS). Neither was the human immunodeficiency virus (HIV) known. That state of innocence has ended forever.”1 In 2000 Larry Kramer, the co-founder of the AIDS Coalition to Unleash Power (ACT UP), reminded the world that the AIDS crisis is more devastating than ever: “Even if we were to find a cure tomorrow, millions and millions of people will die. You were all told this before it was too late. Now it is too late. So sit back and watch the destruction of the world.”2 This may appear melodramatic; however, the message was confirmed at the International Conference on AIDS in South Africa in 2000, where concerns over the expansion of the epidemic were discussed. The United Nations Programme on HIV/AIDS (UNAIDS) estimates that globally, nearly 22 million have died of AIDS and more than 36 million are living with HIV or AIDS. “HIV disease is reversing decades of public health progress.”3 The emphasis of the conference was more on reducing stigma and increasing access to care than on biomedical research.
HIV is an epidemic that must be understood within its historical perspective. In June 1981 the Centers for Disease Control and Prevention (CDC) reported through its Morbidity and Mortality Weekly Reports (MMWR, the disease status and policy reports of the CDC) that five young homosexual men had required treatment for Pneumocystis carinii pneumonia, an opportunistic infection previously seen almost exclusively in immunodeficient patients such as transplant recipients and those under treatment for cancer. Its occurrence in five previously healthy individuals without a clinically apparent underlying immunodeficiency was unprecedented. These men also had cytomegalovirus (CMV) infection and oral candidiasis, further indications that they had a “cellular-immune dysfunction relative to a common exposure that predisposes individuals to opportunistic infections.”4 One month later the CDC reported the occurrence over a 30-month period of an uncommon malignancy, Kaposi’s sarcoma, “among (26) previously healthy homosexual men.”5 The CDC noted that the clinical characteristics of these cases differed from those usually seen with Kaposi’s sarcoma, which had generally been regarded as a disease of elderly men. Again, the situation suggested a common underlying factor—immune suppression.6
In 1984 evidence implicated a retrovirus as the etiologic agent of AIDS, and two prototypes were isolated: lymphadenopathy-associated virus (LAV) in France and human T-cell lymphotropic virus type III (HTLV-III) in the United States, which were later shown to represent the same virus. In 1985 a serologic test became available to detect the presence of antibody to HTLV-III/LAV.7 The availability of this test had varied consequences. First, it permitted investigation of the prevalence of the virus, and these studies demonstrated that infection with the virus itself was more common than the clinical illness (AIDS) in populations with an increased incidence of AIDS. Second, serologic testing gave an opportunity to study the progression of the disease within populations. Third, with the ability to detect antibodies, it became possible to screen blood and plasma donations for the virus. Surveillance of health care workers exposed to the virus also became possible.
Thus in June 1982 there was a hypothesis that a sexually transmitted infectious agent was causing disease in homosexually active men.8 Since then, the landscape has changed considerably. There is an identified agent, a case definition, and a realization that groups other than men who have sex with men (MSM) are being affected. Because of this realization, the focus of prevention has changed from risk groups such as MSM and intravenous (IV) drug users to risk behaviors such as MSM, IV drug use, and multiple sex partners. Clinically well-defined signs and symptoms exist, some of which are oral. Markers are available for the disease, treatments exist, and tests have been developed to measure viral load. Unfortunately, no cure or vaccine exists.
In 1993 the CDC proposed a change in the case definition of AIDS to more realistically measure the extent of disease. The new case definition was defined by a T-cell count of 200 or less and the presence of specific AIDS-defining conditions. These were expanded from the original definition to include, for example, cervical cancer and other conditions that would capture groups without previously recognized symptoms. Although this was important from a surveillance standpoint for tracking disease, it was also important to individuals in terms of becoming eligible for medical benefits that required an AIDS diagnosis. Therefore the increase in AIDS cases in that year was more a product of a change in reporting than an overall increase in HIV incidence.9
The HIV antibody test was licensed in 1985.10 This test detects the presence of antibodies to HIV, not the virus itself. The test thus indicates only that infection with HIV has occurred, with no implications as to health status. A person who has antibodies to HIV is referred to as seropositive, and one without detectable antibodies is termed seronegative. Seroconversion is said to have occurred when an individual’s test becomes positive after some time previous to which test results had been negative. It is important to note that there is a window period during which a person may be infected but during which the body has not yet responded to the virus with detectable antibody response. This time period can range from 3 to 12 weeks after infection, although reports of 6 months or more have been made. The screening test used is the enzyme-linked immunosorbent assay (ELISA). If a specimen has a positive result, a repeat ELISA is generally performed. A persistent positive result is confirmed by a Western blot test. Although both the ELISA and the Western blot test for antibody, the Western blot is considered a more sensitive assay. Therefore persons testing negative on the ELISA are considered seronegative (at least for that point in time), and persons with a positive test confirmed by a Western blot are considered seropositive. For those in whom the results are indeterminate, the tests are repeated.
The Food and Drug Administration (FDA) approved the first antigen test kit in 1996 for use in screening blood donations.11 Although it may take up to 3 months or more to detect antibodies to HIV, antigens, the virus’s own protein, may be detected an average of 6 days earlier. Transfusion-related HIV infection is low. Current tests fail to detect only 1 in 450,000 to 660,000 HIV-positive donations. This represents approximately 18 to 27 donations per year. Use of antigen testing prevents 5 to 10 cases (approximately 25% of current cases of transfusion-associated HIV) per year. Antigen testing is approved only for blood screening and not as a diagnostic tool. Nucleic acid testing can detect minute amounts of viruses such as HIV and hepatitis C virus even earlier and allow detection of contaminated blood in less than 20 days.12
In 1996 the FDA licensed the first home test for HIV antibody.13 Some controversy surrounds this test because in a majority of states and under the professional recommendations, testing should not occur without appropriate counseling before and after the test. That first test was an over-the-counter specimen collection kit in which the user mails a dried blood sample to a laboratory for analysis, with results available after 1 week by telephone. The reported sensitivity is 99.9% and the specificity close to 100%. Therefore 1 in 1000 false-positive results and almost no false-negative results would be expected. Also licensed by the FDA in 1996 is the first saliva-based HIV test. The accuracy of this test is close to that of tests using blood samples.
The FDA has also approved tests that measure the concentration of HIV in the blood, a more appropriate predictor of the progression of disease than previously available tests such as those that measured CD4 counts. Studies on these viral load tests have shown that individuals with levels below 10,000 viral units per milliliter of serum were more likely to survive the 6-year study period than were those with higher levels of virus. The FDA is also reviewing rapid testing for HIV, but no product is yet approved. The CDC has called for faster FDA approval because earlier results may reduce the rate of new infections. The failure of many individuals to return for HIV test results could be minimized by a rapid test. Approximately one fourth of those tested, or 10,000 people annually, do not return for their results.14 A phenotypic HIV drug resistance test exists that can detect the emergence of drug resistance before significant increases in viral loads of patients in treatment. The clinical significance is that modifications in treatment can be made earlier as signs of treatment failure are detected. This could become a standard monitoring tool.15
As of December 1999, more than 733,734 cases of AIDS and 438,795 deaths had been reported to the CDC, and the estimate of those infected with HIV (HIV seropositive without signs and symptoms of AIDS) is 600,00 to 900,00 in the United States, approximately 20,000 of whom are unaware of their infection.16 It took 9 years for the first 100,000 cases to be reported but less than 2 years for the second 100,000 cases. By the end of 1992, 100,000 people had died of AIDS, and there is an AIDS death approximately every 15 minutes. Although the HIV epidemic still affects men who have sex with men more than other groups, the spread of disease into the heterosexual population is becoming more evident. In 1992 the number of women infected through heterosexual contact exceeded the number infected by IV drug use. In 2000 40,000 new cases were reported, and 30% of the new cases were among women. Among men, 60% of the new cases were in men who have sex with men and 15% in heterosexual men. Although the number of new cases steadily declined in the past few years, the slowdown has been reduced for reasons such as complacency about prevention and poor access to treatment. The CDC national goal is to reduce the number of new infections to 20,000 by 2005.17
Newly released figures from the United Nations indicate that 34.3 million people worldwide are either HIV positive or have an AIDS diagnosis. Africa accounts for more than two thirds of the global epidemic, and transmission there is primarily heterosexual.18 It has been suggested that the HIV pandemic ranks as “one of the most destructive microbial scourges in history.”19 Approximately 16,000 new infections occur daily, and more than 95% of these occur in developing countries.
Epidemiologic studies have shown an increase in infection rates among IV drug users and disproportionate rates among minority groups. In Living with AIDS, the National Commission on AIDS reported, “As of June 1991, women accounted for 10% of all AIDS cases … cases among women are growing faster than AIDS cases among men.”20 The rate of new infections has increased in women and individuals of color, who now represent 67% of newly diagnosed AIDS cases, 62% of those living with AIDS, and 69% of new HIV infections. The highest rates are in African-American men and women. African-American and Latina women account for approximately 80% of cases among women.21
Children are becoming infected as well. Nearly 70% of all pediatric AIDS cases are related to the mother’s exposure to the disease. Clinical trials reported in 1994 demonstrated that the use of zidovudine (AZT) can reduce by two thirds the risk of HIV transmission from women to their unborn children. The perinatal transmission rate for women taking AZT was 8.3% compared with 25.5% for those taking a placebo. Vertical transmission rates have steadily declined from more than 20% between 1985 and 1990 to 6.5% between 1990 and 1997. This decline is attributed to the routine treatment of pregnant women with zidovudine. Estimates in 1994 were that between 6000 and 7000 women infected with HIV gave birth in the United States each year and that 1500 to 2000 of the infants were infected. An FDA advisory panel has recommended that AZT be given to all HIV-infected women during specified prenatal and postnatal periods.22 This also has implications for postexposure management in those dental health care workers (DHCW) who are pregnant, although no recommendations have been proposed.
GUIDELINES AND REGULATIONS REGARDING TRANSMISSIBLE DISEASES
In 1970 Congress passed the Occupational Safety and Health Act, creating, within the Department of Labor, the Occupational Safety and Health Administration (OSHA).23 The charge to OSHA was to protect workers and ensure healthful working conditions for every worker in the United States. This act required all employers to provide to all employees “a workplace that is free from recognized hazards that are causing or likely to cause death or serious physical harm.” Before finalizing the bloodborne pathogens rule on December 6, 1991, OSHA relied on this general duty clause to enforce the use of recommended guidelines to control the spread of bloodborne disease among health care workers.24 OSHA is a regulatory agency with enforcement authority and an ability to make citations and impose fines or penalties for failure to comply with established standards, especially when this failure results in illness, injury, or death.
Centers for Disease Control and Prevention
The CDC is an advisory agency with an intent to protect and promote the health of the public. The CDC has no regulatory authority, and the guidelines issued are for the most part voluntary, although over time these guidelines become standards of practice and, in some cases, the basis for regulation by agencies such as OSHA. Once the CDC published its first infection control guidelines in 1982, standards of care evolved for the dental profession.25 The early guidelines did not specifically address dental care but outlined suggested precautions to be used when dealing with patients with AIDS, such as the use of gloves, refraining from bending or recapping needles, the use of gowns, and the use of extraordinary care to prevent injury. In general, early in the epidemic the CDC, reasoning by analogy, recommended use of procedures already known to be appropriate for persons infected with HBV. The first actual recommendations for DHCWs in 1983 stated the following:
State-of-the-art infection control guidelines for dentistry did not emerge until April 18, 1986, when the CDC published “Recommended Infection Control Practices for Dentistry.”27 These recommendations were based on the use of a common set of infection-control strategies to be used routinely in the care of all patients in dental practices. This represented a shift to Universal Precautions from selective precautions. Of special interest is the editorial note that “all DHCWs (dental health care workers) must be made aware of sources and methods of transmission of infectious diseases.”27 It was emphasized that disease transmission in either direction (patient to DHCW or DHCW to patient) could be minimized by following the infection-control guidelines. In addition, vaccination for HBV was strongly recommended for dental personnel as a supplement to, not a replacement for, strict adherence to Universal Precautions.
The guidelines for dentistry, updated and released in July 1993, represent a logical progression of knowledge in the emerging science of infection control and exposure management.28 The new recommendations emphasize behavior-driven components of exposure control and issues of patient safety, including providing a clear understanding of disease transmission mechanisms and associated risk. The overall premise is the same as in 1986, that “dental patients and DHCWs may be exposed to a variety of microorganisms via blood or oral respiratory secretions…. Infection via any of these routes requires that all three of the following conditions be present: a susceptible host; a pathogen with sufficient infectivity and numbers to cause infection; and a portal through which the pathogen may enter the host. Effective infection control strategies are intended to break one or more of these links in the chain, thereby preventing infection.”28 In addition, there is a shift from the earlier emphasis on bloodborne disease transmission to now include airborne disease concerns, such as Mycobacterium tuberculosis and other upper respiratory illnesses. These new recommendations are intended to provide direction where there is no current regulation from OSHA.
Other major guidelines for infection control were released in 1987 and 1988 from the CDC and referred, in part, to all health care workers but also, in part, specifically to DHCWs.29,30 The CDC now made note of the fact, cited previously, that the antibody status of most patients would not be known; therefore these recommendations were to apply to all patients and all health care workers who performed or assisted in invasive procedures. The 1987 guidelines were the first to introduce the concept of Universal Precautions. All previous recommendations were selective precautions. The recommendations included the wearing of gloves and other personal protective barriers such as masks and eyewear, the handling of needles and other sharp instruments in such a manner as to prevent injury, and the management of specific exposure incidents with a potential for disease transmission. In the recommendations specific to dentistry it was emphasized that gloves were to be regarded as single-use items. Handwashing; the use of masks, protective eyewear, and gowns where indicated; disinfection of environmental surfaces; and sterilization of instruments were more fully defined. The precautions recommended for dentistry began to recognize that blood, saliva, and gingival fluid should be considered infective. Handpiece sterilization and infection control procedures for dental laboratory cases emerged as important issues for dentistry. All of these recommendations became the basis for the OSHA bloodborne pathogens standard.
RISK TO DENTAL HEALTH CARE WORKERS
The first case of occupationally acquired HIV infection, by a needlestick, was reported in Africa in 1984. It also became apparent during this period (1988 to 1990) that DHCWs were themselves susceptible to becoming infected.31 Indeed, two dentists were reported to have most likely seroconverted as a result of occupational exposure.
As of December 31, 1999, 56 health care workers had been reported to the CDC as having a documented occupational transmission of HIV, through a special study set up to monitor health care workers.32 Documented means that seroconversion occurred after an occupational exposure to blood known to be infected with HIV. The exposed DHCW must also have had a baseline HIV test that was negative at the time of exposure, and seroconversion must have occurred 3 to 6 months later. Among the documented cases, 48 reported percutaneous exposures; 5 reported blood splashes to the eyes, nose, or mouth; 2 reported percutaneous and mucocutaneous exposure; and 1 reported an unknown route. Forty-nine were exposed to blood of an HIV-infected person, 1 to visibly blood-contaminated bodily fluid, 3 to an unspecified fluid, and 3 to concentrated virus in a laboratory. Subsequently, AIDS has developed in 24 of these health care workers.
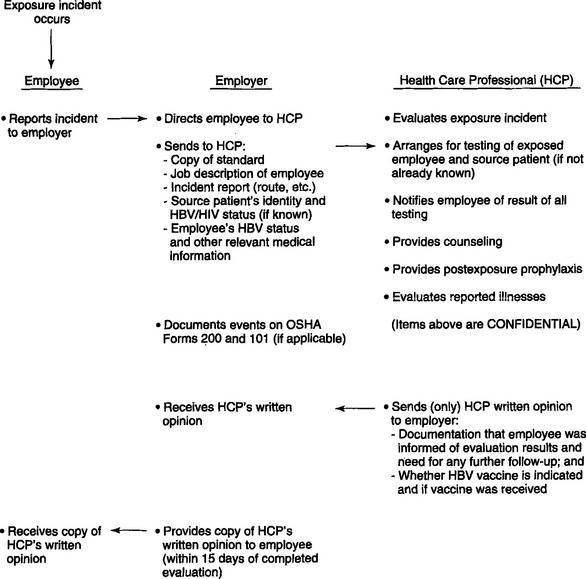
One hundred thirty-eight health care workers have been reported to the CDC as a “possible occupational transmission,” and among these are six dental workers. These health care workers have been investigated and are “without identifiable behavioral or transfusion risks; each reported percutaneous or mucocutaneous occupational exposures to blood or body fluids, or lab solutions, but HIV seroconversion specifically resulting from an occupational exposure was not determined.”32 Occupational exposure control is a serious issue. In February 1995, OSHA issued a guide to dental employer obligations as a follow-up to the bloodborne pathogens standard in regard to occupational exposure.33 These detail, in a step-by-step guide to compliance, the recommendations and regulations regarding management of exposure incidents in oral health facilities (Fig. 9-1). The employer is obligated to provide, not perform, a confidential medical evaluation and follow-up by a licensed health care professional at no cost to the employee.
Among the medical services that the employee must be offered are counseling, collection and testing of the employee’s blood, postexposure prophylaxis (in accordance with U.S. Public Health Service recommendations), and evaluation of reported illnesses. The employer is obligated to pay only for the cost of treating the incident and not the cost of the subsequent disease should seroconversion occur. All exposure incidents are recordable for purposes of OSHA’s record-keeping requirements. Testing of source patients is in accordance with state laws and with consent of the patient. The U.S. Public Health Service did not officially recommend zidovudine for HIV postexposure prophylaxis until recently. OSHA therefore did not require the employer to pay for it.34 It would be prudent to assume that OSHA would expect the employer to now pay for the antiviral regimen because there have been changes in the U.S. Public Health Service recommendations. Previously the U.S. Public Health Service neither recommended nor advised against the use of zidovudine for postexposure prophylaxis. However, the agency did recommend offering it to an exposed health care worker. Findings released by the CDC in December 1995 associate the use of zidovudine with a lower risk for HIV transmission after studying its use in health care workers who sustained percutaneous injuries to blood known to be infected with HIV.35 The data suggest that use of zidovudine postexposure may be protective for health care workers. The study investigated factors associated with HIV transmission, such as exposure to a large quantity of blood, a deep injury, a visibly contaminated device, and terminal illness in the source patient. Risk for HIV infection among health care workers who used zidovudine was reduced approximately 79% with other factors controlled. These results prompted the U.S. Public Health Service to evaluate the data and to propose revisions for postexposure management (PEM) on the basis of the severity of an injury and the HIV status of the source patient. Newly released recommendations for PEM of individuals exposed to blood known to be infected with HIV include the use of antiretrovirals, either as monotherapy or in combination as determined by source individual and injury information.36 The current U.S. Public Health Service recommendation is that health care workers who are exposed to HIV on the job should, in many cases, take zidovudine and other antiretroviral drugs after exposure to reduce their risk of becoming infected.
Injury data on DHCWs has been derived from observational, retrospective, and prospective studies (including self-reported data). In 1987 dentists reported approximately 1 injury per month (12 per year). By 1991 dentists reported 0.3 per month, or approximately 3 to 4 per year. More recent data from the CDC indicate a further decline to approximately 0.18 per month, or 2 to 3 per year. This supports the assumption that most injuries are preventable with appropriate administrative, engineering, and work practice controls. Most percutaneous injuries occurred outside the patients’ mouth, most on the hands of the dentist. Burs were the most common source (37%), followed by syringe needles (30%), sharp instruments (21%), and orthodontic wires (6%). The CDC stated that “the rate of percutaneous exposures among dental workers … is probably less than among general surgical personnel. Most injuries are outside the mouth, involve the fingers and hands and are self-inflicted.”37
Of a total population of more than 213,357 professionally active dentists and dental hygienists, the current estimate of dentists and dental hygienists with AIDS is only 467, of which approximately 374 have died.16 There are possibly more than 2000 DHCWs with HIV. The best estimates of risk to health care workers is 0.3% for HIV transmission from percutaneous exposures and 0.09% for mucous membrane exposures (even less for skin contacts), 3% to 10% for hepatitis C virus (HCV), and 30% for HBV transmission after percutaneous injury from an infected patient.38 From the data available, it appears that the risk of HIV infection to DHCWs is extremely low. Health care workers represent approximately 5% of the general population and approximately 5% of reported AIDS cases, indicating that they are not overrepresented.
RISK TO PATIENTS
DHCWs had already recognized the potential to transmit disease in either direction. HBV transmission had been well documented from dentists to patients, as well as herpes transmission from dental hygienists to patients. Transmission of HBV from dentists to patients has not been reported since 1987. Health care workers, primarily physicians and dentists, have a threefold to fivefold higher prevalence of HBV than the general population does. However, transmission from HIV-infected dental health care workers to patients had not yet been reported. Since the early 1970s, when serologic testing became available for HBV, the CDC had reported on 20 clusters of HBV transmission to more than 300 patients from infected health care workers. In 12 of the clusters the health care worker did not routinely use gloves, and some reported skin lesions that could have promoted the transmission. Nine of these clusters were linked to dentists or oral surgeons. Many of the transmissions could have been prevented by strict adherence to current Universal Precautions. Most of the reports were before the acceptance of Universal Precautions. The CDC suggested that “the limited number of reports of HBV transmission from HCWs [health care workers] to patients in recent years may reflect the adoption of Universal Precautions and increased use of HBV vaccine.”39
Previous experience with HBV transmission suggested that the performance of invasive procedures was more likely to contribute to disease transmission, that the use of Universal Precautions was likely to reduce the risk of transmission, and that this transmission would be expected to “occur only very rarely.”40 Therefore routine testing of health care workers was not recommended. Recent reports of HBV transmission from a health care worker to patients during performance of invasive, exposure-prone procedures are not among dentists, but refer to a surgeon who is HBeAg positive and had not been vaccinated against HBV. Approximately 1% of surgeons are infected with HBV, and transmission is thought to be rare.31 In 1992 a female patient was diagnosed with HBV, and transmission was associated with cardiac surgery. No deficiencies in infection control were detected and no specific events could be identified. HBV was transmitted to at least 19 patients studied, and epidemiologic and laboratory evidence “support the surgeon as the source of infection.” Factors of transmission were more likely related to irritations on the surgeon’s fingers, and virus, which may have escaped through tiny holes in the gloves, was found in the glove washings. Health care workers who are positive for HBeAg are more infectious, and reports of transmission of HBV since the early 1970s have been associated with this state.
Hepatitis C virus (HCV) transmission in health care facilities has also been documented. In a study conducted from 1992 to 1994, five patients of a cardiac surgeon were identified in whom infection may have been transmitted by the surgeon. All were infected with the same HCV genotype. The surgeon reported one serious percutaneous exposure from a patient with HBV and was treated for this exposure. Overall, the surgeon reported a rate of approximately 20 percutaneous injuries per 100 procedures, most of which went unnoticed until after the procedure.41 This resembles reports of HBV transmission in surgical and dental settings as a cluster of cases. Simultaneous transmission of HIV and HCV to a health care worker who sustained a deep needlestick injury from an HIV/HCV-infected source patient in 1990 was reported. Use of zidovudine was declined. This is the first report of simultaneous transmission.42 Researchers at the International Conference on Emerging Diseases convened in 2000 reported that health care workers had a 20 to 40 times greater risk of contracting HCV from an accidental needlestick than HIV. A study of 66 hospitals in South Carolina indicated that 5.2% of health care workers were infected with HCV compared with 2.3% with HIV.43
When the inevitable became the actual with the first report of transmission of HIV from an infected health care worker to a patient during an invasive procedure, it was indeed in dentistry.44 The first report of a “possible” transmission to “patient A” came in the July 1990 issue of the CDC’s Morbidity and Mortality Weekly Report.45 By January 1991 the transmission was no longer considered merely possible, and the report then read, “Update: Transmission of HIV Infection during an Invasive Dental Procedure.”46 The concept of transmission from health care worker to patient had progressed from highly improbable to possible to probable in less than a year, and the involvement had increased from one to six patients.47 These events, leading up to the death of patient A, Kimberly Bergalis, left an indelible imprint on dentistry.
GUIDELINES FOR HUMAN IMMUNODEFICIENCY VIRUS/HEPATITIS B VIRUS—INFECTED DENTAL HEALTH CARE WORKERS
The public outcry over the “first real victim of AIDS” was deafening. Conservative congressmen called for stiff measures, from jailing infected health care workers who continued to practice to mandatory testing of all health care workers. One of the most serious possible consequences of this event for the health care field in general could be the loss of the professional control over the future of health care workers. At a minimum, proposals ranged from reviewing the health status of infected health care workers (HIV and HBV) by expert review panels to mandatory testing and patient notification. By an act of Congress in October 1991, states were given 1 year in which to adopt the CDC “recommendations for preventing transmission of human immunodeficiency virus and hepatitis B virus to patients during exposure-prone invasive procedures” or else to come up with their equivalent and have it approved by the CDC; all states have complied.48 In essence, the CDC recommendations cited by Congress are based on a series of assumptions as to the likelihood of transmitting disease from infected providers to patients:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses