9
Materials in restorative dentistry
- The properties, chemistry and clinical handling of materials for direct tooth restoration
- Amalgam
- Resin composite
- Glass ionomer cements
- Resin-modified glass ionomer cements
- Bonding to enamel and dentine
- Pulp protection regimes
- An overview of impression materials in common usage
Introduction
The purpose of this chapter is to consider the restorative materials available for the direct restoration of permanent and deciduous teeth. The chemistry and physical properties, as they relate to modern-day restorative practice, will be discussed, and the advantages, disadvantages, indications and contraindications of the materials will be outlined. To assist the reader further, hints and tips that relate to the successful use of these materials will be described.
Amalgam
Amalgam is one of the oldest direct restorative materials that are still in regular use. Relatively few changes have occurred in the recipe for amalgam since G.V. Black described a formulation that was durable and reliable, although the changes that have been made have significantly improved the physical properties of amalgam. For the most part of its historical use in dentistry, there have been few alternative direct placement materials, and even today amalgam is still considered by many to be the best available material to fill large cavities in load-bearing teeth.
An amalgam is a metal alloy containing mercury and one or more other metals. In the case of dental amalgam, the other metals are typically silver, tin and copper (Table 9.1). There may be traces of other metals included to modify the properties of the material, or aid in its manufacture.
Table 9.1 Typical composition of a modern amalgam powder.
| Component | Percentage composition |
| Silver | 60–70 |
| Tin | 27 |
| Copper | 13 |
| Zinc | 0–2 |
The powdered alloy is mixed with liquid mercury, which readily reacts with the metals incorporated into the powder. Mercury is liquid at room temperature, and will give off a vapour which is potentially hazardous. To prevent inadvertent exposure it is usual for the powdered alloy and liquid mercury to be packaged in predetermined amounts in a sealed capsule (Figure 9.1). The powder and liquid are kept apart during storage by a thin membrane that is broken on activation. Alternatively, the powder and liquid could be stored in two hoppers in an amalgamator, which are then dispensed in a ratio and amount that are controllable by the operator. Amalgamators require the topping up of powder and mercury from time to time and this process increases the chance of mercury spillage. The mercury needs to be very pure and is usually triple distilled to eliminate impurities.
Figure 9.1 Schematic diagram of an amalgam capsule. The capsule is activated by pressing in the plunger shown in green. This breaks the membrane, allowing alloy and mercury to be mixed together. The end of the capsule containing the powder is detachable, and can be used as a receptacle for the set material.
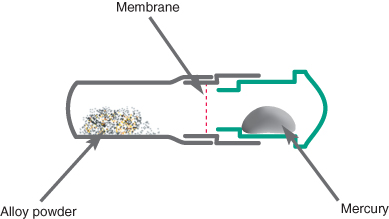
The final set material should contain 45–50% mercury; however, when there is less than 50% mercury in the amalgam mix it can prove too dry and difficult to work with. The more mercury in the mixed material, the softer the material is and the easier it is to pack and carve, but the set restoration will be weaker and more prone to corrosion. To reduce the amount of mercury in the final restoration, the amalgam should be vigorously condensed, as this causes excess mercury to rise to the surface where it can be carved away and discarded in a safe manner. For this reason amalgam should always be placed to overfill a cavity.
Types of amalgam
Amalgam may be classified by the shape of the particles that make up the powder, or the constituent metals of the particles. The particles of alloy that make up the powder can either be spherical in shape or an irregular shape known as lathe-cut (Figure 9.2). The shape of the particles determines how the material handles. Spherical particles are formed by spraying molten alloy into an inert atmosphere. As it falls spheres form and solidify. The other method of creating the powder is to grind small particles from a solid block of alloy, hence the name lathe-cut. It gives more resistance when packing into cavities than spherical amalgam, but is not as prone to slump when building up a large restoration.
Figure 9.2 Types of particles available in common usage.
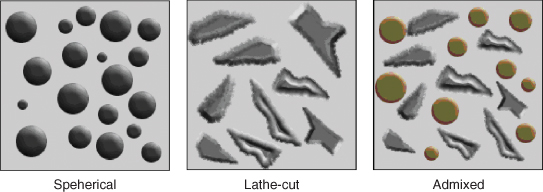
One of the most popular types of amalgam in current usage is dispersed phase or admixed amalgam, consisting of spherical silver/copper particles and lathe cut silver/tin particles. Combined together, they give good handling characteristics. With spherical amalgams being more easily condensed, less mercury is needed to wet the particles, which in turn leads to a final material with lower mercury content and better physical properties.
Setting reaction
The silver/tin phase, Ag3Sn or γ phase, in the powdered alloy reacts with mercury when mixed. The mercury will dissolve the outer layer of particles, allowing the release of the metal ions within. Smaller particles will completely dissolve, but larger particles often keep their cores intact. The metal ions released join to form grains which grow until eventually the material becomes solid (Figure 9.3).
Figure 9.3 The setting reaction for traditional dental amalgam. There will be an amount of γ phase remaining in the final material, as not all of each particle is dissolved into the mercury. The γ2 phase has adverse effects on the properties of the material.

The set material contains unchanged particle cores consisting of the gamma (γ) phase, surrounded by a matrix of γ1 and γ2. The γ2 phase is associated with increased corrosion, creep (plastic change over time) and lower strength. Modern amalgams have low γ2 content due to the presence of higher amounts of copper. The copper acts to convert the γ2 phase to γ1 and the formation of a silver–copper alloy, Cu6Sn5, by the reaction:
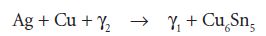
The copper can either be mixed with the alloy powder, so that the particles contain silver, tin and copper, or silver–copper particles can be added to a traditional amalgam powder. For this reason modern amalgams are known as ‘high copper’ amalgams or ‘non gamma-2’. On initial set, the material is quite weak and prone to fracture if heavy occlusal forces bear down on it, and it takes up to 24 hours for the material to reach its optimal strength.
Properties of amalgam
Good compressive strength gives amalgam the ability to withstand the forces of biting and chewing. It is hard wearing, yet kind to the tooth it is placed in and causes very little wear on the teeth opposed by it, due to low abrasivity (Jagger and Harrison, 1995).
Corrosion is reduced with the addition of copper but is still responsible for tarnishing giving a dull appearance and fatigue particularly at amalgam margins leading to marginal breakdown. Corrosion products however can be beneficial as they have been shown to seal the interface between the amalgam and the tooth reducing microleakage.
Clinical usage
Cavity preparation
Amalgam does not chemically bond to tooth surface but requires tooth preparation to create a shape of cavity that contains both retention and resistance forms to prevent dislodging of the restoration. This is carried out by creating undercuts, dovetails, pits and grooves in the dentine of the tooth. This inevitably requires more tooth tissue to be removed than is necessary to remove the caries alone and can lead to unnecessary destruction of precious tooth tissue. In addition to this, amalgam is weak in thin section, so cavities need to be created that are at least 1.5 mm deep and where the cavo–surface angle is never below 90°.
During the condensation stage of placing amalgam, the material is firmly pressed into all recesses of the cavity preparation. The act of vigorous condensation also brings any excess mercury in the material to the surface. As each successive increment is added the mercury can be encouraged to rise successively. The final increment should overfill the cavity and the now mercury rich top layer is removed during the carving process and discarded safely.
Carving should be carried out while the material is soft, using hand instruments to recreate the anatomical features of the tooth. It is important to remove any excess material overlying the enamel surrounding the margins of the cavity otherwise once set it will come under occlusal load and being in thin section will fracture and leave a positive margin to the restoration ultimately acting as a plaque retention factor and area of stagnation. The amalgam–margin angle following carving should be no less than 70° to avoid thin sections as the material tapers out towards the edges of the cavity which could in time fracture off leaving a negative restoration margin with similar consequences.
Burnishing the material can improve the marginal adaptation and achieve a surface smooth enough without the need for polishing which is carried out less frequently than it was in the past. Detractors of polishing claim that shiny spots caused by heavy occlusal contacts are less easily recognised on a polished surface.
Amalgam bonding
The major drawbacks of amalgam are its very poor aesthetics and the fact that it does not bond to the tooth; hence its use does not encourage conservation of tooth tissue or prevent marginal microleakage. Recent developments in bonding techniques using materials that can both bond to the tooth and bond to the amalgam itself have reduced the need for dentine pins in larger cavities. There are several materials commercially available suitable for bonding amalgam. They contain chemically active resins such as 4-methacryloyloxyethyl trimellitate anhydride (4-META). The use of bonding systems, whilst potentially advantageous, adds a further step to the restoration process and the materials are highly technique sensitive. On top of this there is no clear evidence that bonding reduces the incidence of secondary caries or improves the longevity of the restoration. A simpler method of bonding amalgam using RMGIC is described later in this chapter.
Indications for amalgam restorations
Amalgam has a high compressive strength, but offers poor aesthetics and so is best suited to restorations of premolar and molar teeth, where heavy occlusal forces are experienced and where the cavities are not suitable for composite resins. Due to their durability, amalgam restorations are often used to rebuild badly broken down teeth prior to final restoration with crowning.
Recent changes in the National Health Service and pressure from patients to use alternative materials that are more aesthetic, or who have concerns about the mercury content of their restorations, may well see an end to the widespread use that amalgam has in today’s dentistry.
Resin composite
Resin composites are a combination of a resin matrix, which is polymerisable, and an inorganic filler. Both components play a part in the final material’s properties and both have been varied in their composition to attain desirable properties for differing situations. Composites are in fact a family of materials that can be utilised in many situations in dentistry where aesthetics, bonding ability and strength are the prime requirements.
Resin matrix
The setting reaction of a resin composite involves the polymerisation of the resin matrix. This is the process whereby small components, termed monomers, combine to form large-chain molecules. The longer the chain, the more viscous the material becomes until it reaches a solid state. The polymerisation process is often described as the setting reaction or curing process. The setting reaction is initiated by the production of free radicals, which are highly reactive charged substances within the material. These initiate the chain reaction of polymerisation. Free radicals can be created in one of two main ways with modern resin composites: by chemical reaction or by light.
Self-curing resin composites, sometimes known as chemically cured or autopolymerising resin composites, set without the need for a light. They are usually presented as two pastes both containing resin and filler. A tertiary aromatic amine has been added to the base material paste, and dibenzoyl peroxide is added to the other (the catalyst). When the amine and peroxide meet, the peroxide is cleaved to form two molecules each with a free radical.
For light-curing materials, there is a single paste containing resin, filler and camphorquinone. The camphorquinone has a breakable bond within it that is broken when exposed to light of about 470 nm. The result of this is the production of free radicals to initiate polymerisation.
Both methods of free radical production have their advantages and disadvantages. Light curing allows the operator to be in control of the working time. The greatest problem with light curing is that sufficient light of the correct wavelength needs to satisfactorily penetrate the material in order to get a high enough degree of polymerisation for the resin composite to have optimal properties. They also can become difficult to handle as they begin to polymerise under the glare of the dental light. In deep cavities and underneath crowns and veneers, chemically cured materials have the advantage in that they will optimally polymerise whatever the depth or availability of light. The largest disadvantage of chemical curing is that there is no control over working time and materials may set sooner than expected.
To try to overcome the problems with both types of curing, ‘dual-cure’ materials were developed. These are partially set by light cure so that control of the working time is maintained but they will undergo a slower chemical cure to permit full curing of the whole restoration.
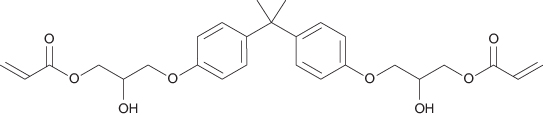
During polymerisation heat is produced and the resin shrinks as the monomer molecules join together. The shrinkage of pure acrylic resin alone can be up to 21%. This amount of shrinkage would have devastating consequences for a tooth, as it would lead to stresses within the enamel and dentine as both sides of a cavity were pulled together. If the bond to tooth tissue was not maintained there would be considerable marginal microleakage, leading to sensitivity and secondary caries. Shrinkage can be reduced by utilising higher molecular weight monomers. The predominant substance used as a monomer in dental resin composites is Bis-GMA (Figure 9.4). Bis-GMA produces a viscous, sticky resin; to help the material flow, smaller molecular weight monomers are added, such as TEGDMA (triethyleneglycol dimethacrylate). These are referred to as diluent monomers.
Figure 9.4 The formula for bisphenol A-diglycidylether methacrylate (Bis-GMA).
Larger methacrylate monomers, such as Bis-GMA, help reduce the shrinkage to a certain extent, but that alone does not reduce it sufficiently for the material to be clinically acceptable. It is the filler particles that reduce the overall amount of shrinkage to a degree that is acceptable, currently about 2%. In addition it is the amount and size of the filler particles that define the categorisation and intended applications for resin composites.
Filler particles
As a general rule, the more filler present, the stronger and harder wearing the resin composite will be, whilst also being less affected by shrinkage. Too much filler, on the other hand, may affect the optical properties and polishability of the material, also more filler makes the material less flowable.
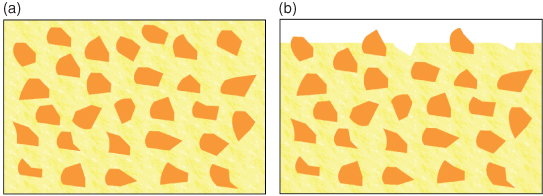
Early resin composites were filled with quartz particles of relatively large size. These are known as macrofilled resin composites. They are strong, but give poor long-term aesthetic results due to the size of the filler particles providing a surface that is not readily polished and which easily takes up staining (Figure 9.5). Filler particles in present use are composed of glasses often containing barium or strontium; these give the material radio-opacity, which helps in the detection of secondary caries.
Figure 9.5 Macrofilled resin composite. (a) The gross structure shows the filler particles embedded within the resin matrix. (b) After wear has occurred, the surface of the material is rough, with particles protruding from the surface and small voids where some particles have been lost.
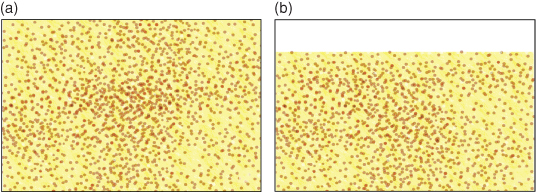
Macrofilled resin composites have a high proportion of filler particles, about 86% loading by volume, which improves wear resistance and increases strength, although, due to the relatively poor aesthetic properties, they are really only suitable for posterior restorations. In order to produce a material that could be polished and retain better aesthetics, a later development was to introduce smaller particles composed of colloidal silica. These are known as microfilled resin composites (Figure 9.6).
Figure 9.6 (a) A single-phase microfilled resin composite containing colloidal silica filler. (b) Following wear, the surface is still smooth and will not stain so readily as the macrofilled resin composite.
During manufacture of microfilled resin composites, difficulty is encountered in obtaining a high enough filler content, to the effect that the resin is only about 50% loaded with filler. This low filler loading has an adverse impact upon the material’s long-term strength and wear resistance. Lower percentages of filler also mean more polymerisation shrinkage with the problems which are associated with it. To try to overcome the problems with both macro- and microfilled resin composites, a hybrid of the two has been developed, containing both larger quartz particles and the smaller silica particles (Figure 9.7a).
Figure 9.7 A hybrid resin composite containing both larger quartz particles and smaller colloidal silica particles (a) compared with the newly developed ‘nano’ composite (b) which, like the hybrid, has larger particles for improved strength and wear resistance; however, each large particle is a cluster of nano-particles that act together, but as the surface becomes worn will separate out individually, leaving a smooth, more stain-resistant surface.
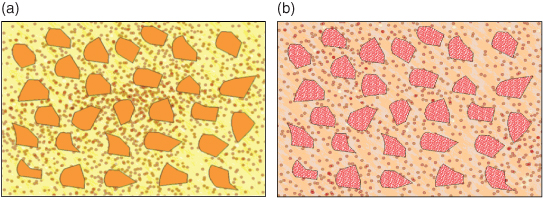
Hybrid resin composites are in common usage as ‘all purpose’ materials, meaning they are suitable for both posterior and anterior teeth. The problem of having larger crystals that can protrude from the surface or be lost still remains, and has recently been addressed by nanotechnology, replacing the larger particles with clusters of nanoparticles (Figure 9.7b). These act together to give the material the strength of a hybrid, but, as wear occurs, they do not leave large voids, allowing the restoration surface to remain polished and stain free.
In all composite materials, to ensure that the resin and filler work together to strengthen the material and avoid loss of surface particles, the two components are joined together by a silane coupling agent.
Flowable resin composites
Flowable composites are a relatively recent addition to the composite family. They have a lower filler content of about 50–70% by volume and are thus less viscous and are able to flow easily into cavities, though their wear resistance is low. The utilisation of monomers with elastic properties has led to the practice of using them to line cavities in posterior teeth prior to restoration with a more conventional composite. The inherent elasticity may to some degree absorb the stresses created during polymerisation shrinkage of the regular composite. Having a lower filler content means that polymerisation shrinkage will be increased. Efforts to reduce the stresses that this increased shrinkage would create upon the tooth include the addition of a modulator that controls polymerisation stress, allowing materials such as Dentsply’s SDRTM to be able to be used as a bulk filler in larger restorations prior to veneering the occlusal surface with a more highly filled material.
Advantages of composite materials
Modern day resin composite restorative materials have considerable advantages for the practitioner and patient, which include the following:
- Used in conjunction with a dentine adhesive system, they can be placed with minimal or no tooth preparation. This is a distinct advantage over amalgam which requires a more destructive cavity design to gain retention and resistance form for the cavity. In this respect the utilisation of composite materials facilitates preservative preparation of teeth when a lesion requires operative management, and also non-destructive management of tooth wear.
- Light curing allows for command cure, which permits immediate finishing and polishing.
- The restoration, if placed correctly in suitably selected teeth, seals the tooth–restorative interface, reducing marginal leakage which can lead to staining, secondary caries and tooth sensitivity.
- It is possible to add material to cured increments, which allows for incremental build-up and further additions at a later date, which means that practitioners can refinish, refurbish and/or repair restorations. Followed to its natural conclusion this will result in a more conservative, less destructive pattern of care.
Disadvantages
Resin composite materials suffer certain limitations, which include the following:
- Polymerisation shrinkage of typically 2–3% can disrupt the marginal adaptation of the material, fracture weakened cusps, notably in premolars, and produce postoperative sensitivity. This can be compensated for to a certain extent by correct placement procedures (see Chapter 13, p.286).
- Bonding to dentine can be problematical, especially at the margins of a preparation (for example, the floor of the box when the floor is below the cemento–enamel junction (CEJ) in proximal preparations).
- Water absorption with surface and marginal staining after some years of clinical service.
- Patient and operator sensitivity to the components of adhesive resins, in particular HEMA.
- Poor radiographic definition (unlike amalgam) making diagnostic interpretation more difficult.
Indications for resin composites
Current indications for resin composites include:
- Small, medium and large occlusal restorations in posterior teeth.
- Small, medium and large proximal restorations in premolar teeth and small to medium proximal preparations in permanent molar teeth. Where the proximal margins are below the CEJ, a bonded-base approach is indicated.
- Cervical lesions in all teeth.
- Incisal edge restorations.
- Fissure sealants and preventive resin restorations.
Contraindications
It is suggested that resin composite should not be used in the following situations:
- Large proximal preparations in permanent molar teeth in patients or areas where they would be subjected to high oc/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


