Locally Delivered, Controlled-Release Antimicrobials
Background and Objectives
It is well accepted that evidence-based practice, providing dental treatment based on research evidence, leads to the most optimal care for the greatest number of patients.66,67 A considerable set of data exists with respect to locally delivered, controlled-release antimicrobials, perhaps the most robust dataset that currently exists for any periodontal therapy. The focus of this chapter is to consider the available clinical data that support the use of these agents for the treatment of chronic periodontitis and to provide an evidence-based guidance for their use. For this discussion, locally delivered, controlled-release antimicrobials are considered as a drug class rather than individually. Since appropriate comparative trials have not been performed, there are insufficient data on which to base any comparison of agents or to consider differential indications for use. The indications for use for each product can only be based on the indications as described in the respective full prescribing information. Additionally, some commentary is provided regarding these agents and available research for potentially new indications that have not yet been evaluated by the U.S. Food and Drug Administration (FDA; Box 89-1).
Currently Available Agents (U.S.)
Three locally delivered, controlled-release antimicrobial products are currently available for dental use in the United States (Table 89-1), a chlorhexidine-containing chip (PerioChip), a doxycycline gel (Atridox), and minocycline microspheres (Arestin). A fourth product, an ethylene/vinyl acetate copolymer fiber containing the antibiotic tetracycline (Actisite; 12.7 mg per 9-inch filament), was the first product introduced into the U.S. market in the early 1990s and was the prototypic system. The tetracycline fiber is no longer commercially available in the United States, and its clinical use will not be further considered. The fiber is included in this chapter, however, because data generated from clinical studies of the product are pertinent for a discussion of the general effects of locally delivered, controlled-release antimicrobials.
TABLE 89-1
Locally Delivered Controlled-Release Antimicrobials Developed for Dental Use in the United States
| Product | Antimicrobial Agent |
| Actisite* | Tetracycline |
| PerioChip | Chlorhexidine |
| Arestin | Minocycline |
| Atridox | Doxycycline |
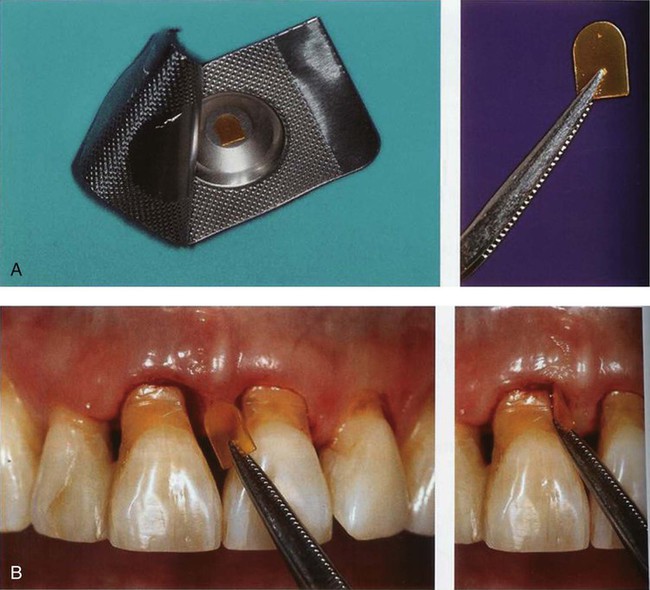
Chlorhexidine Chip.
The chlorhexidine chip is a small chip (4.0 × 5.0 × 0.35 mm) that contains 2.5 mg of the active ingredient chlorhexidine gluconate in a resorbable, biodegradable matrix of hydrolyzed gelatin, cross-linked with glutaraldehyde, packaged in individual foil containers (Figure 89-1, A). It is indicated as an adjunct to SRP procedures for the reduction of pocket depth (PD) in patients with adult periodontitis and may be used as part of a periodontal maintenance program, which includes good oral hygiene and SRP.80 After placement in the pocket, the chip has been reported to release chlorhexidine into the gingival crevicular fluid (GCF) over 7 to 10 days.96 Chlorhexidine is active against a broad range of microbes, disrupting the cell membrane and causing precipitation of the cytoplasm, resulting in cell death. No adverse alterations within the oral microbial flora or overgrowth of opportunistic microorganisms have been observed.80 The chip is biodegradable and does not require removal, but dental floss should be avoided for 10 days to avoid dislodging the chip. The chip is stored at 20° to 25° C (68° to 77° F), with excursions permitted to 15° to 30°C (59° to 86°F). The chlorhexidine chip is placed into the pocket directly from the foil container using a forceps (Figure 89-1, B).
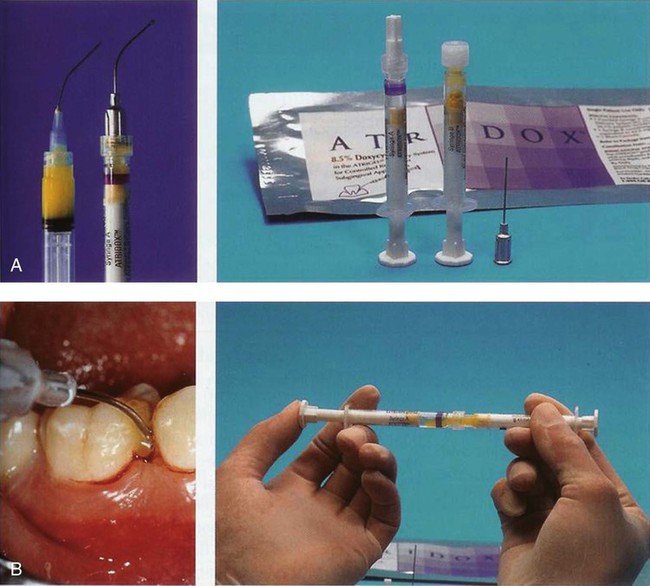
Doxycycline Gel.
The doxycycline gel is a subgingival controlled-release delivery product composed of a two-syringe mixing system (Figure 89-2, A). Syringe A contains 450 mg of a bioabsorbable polymeric formulation of 36.7% poly(D,L,-lactide) dissolved in 63.3% N-methyl-2-pyrrolidone. Syringe B contains 50 mg of doxycycline hyclate, equivalent to 42.5 mg doxycycline. The two syringes are stored at 2° to 30° C (36° to 86° F). When mixed, the constituted product is a viscous liquid of 500 mg, which contains 50 mg (10%) doxycycline hyclate. The doxycycline gel is indicated for the treatment of chronic adult periodontitis for a gain in clinical attachment, reduction in probing depth, and reduction of bleeding on probing.8 Doxycycline, a broad-spectrum semisynthetic tetracycline, is bacteriostatic, inhibiting bacterial protein biosynthesis by interfering with transfer RNA (tRNA) and messenger RNA (mRNA) at the ribosome. No overgrowth of opportunistic organisms was observed following use of the doxycycline gel.8 The gel has been reported to release doxycycline within the GCF over 7 days.103 The doxycycline gel is biodegradable and does not require removal. If not used immediately, the mixed contents in syringe A may be stored at room temperature for a maximum of 3 days within an airtight container. The doxycycline gel is used by injecting the mixed contents of the two syringes directly into the pocket (Figure 89-2, B). The pocket contents are then covered with either a periodontal dressing or a cyanoacrylate dental adhesive.
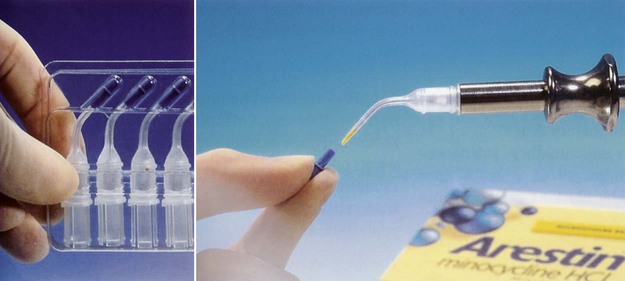
Minocycline Microspheres.
The minocycline microspheres product is a subgingival controlled-release delivery system containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable poly(glycolide-co-D,L-lactide) polymer in unit-dose cartridges (Figure 89-3). Each cartridge delivers minocycline hydrochloride equivalent to 1 mg of minocycline free base. The minocycline microspheres are indicated as an adjunct to SRP for reduction of PD in patients with adult periodontitis and as part of a periodontal maintenance program, which includes good oral hygiene and SRP.6 Minocycline belongs to the tetracycline class of antibiotics and has a broad spectrum of activity. Its bacteriostatic, antimicrobial activity results from the inhibition of protein biosynthesis. No overgrowth of opportunistic microorganisms was noted during clinical studies. No changes in the presence of minocycline-resistant bacteria, Candida albicans or Staphylococcus aureus, in the gastrointestinal tract were detected at the end of a 56-day study, although there was a slight increase in the numbers of minocycline-resistant bacteria in periodontal pocket plaque samples after 9 months.6 The clinical significance of the changes is unknown. Patients should avoid hard or sticky foods at the treated teeth for 1 week and interproximal cleaning devices for about 10 days. The minocycline microspheres formulation is biodegradable and does not require removal. The cartridges are stored at 20° to 25° C (68° to 77° F), with excursions permitted to 15° to 30° C (59° to 86° F). Excessive heat should be avoided. The minocycline microspheres are used by injecting the contents of a unit-dose cartridge into the pocket. Neither a periodontal dressing nor an adhesive are needed.
For complete details for the use of locally delivered, controlled-release antimicrobial products, including a discussion of the safety profiles, the reader is referred to the full prescribing information for each product.6,8,80
Rationale for Local Delivery and Controlled-Release
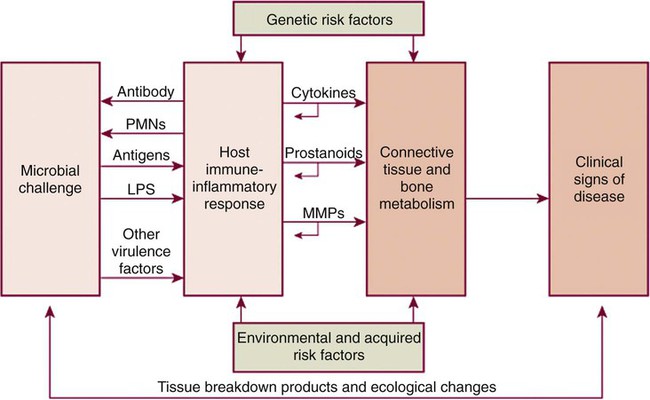
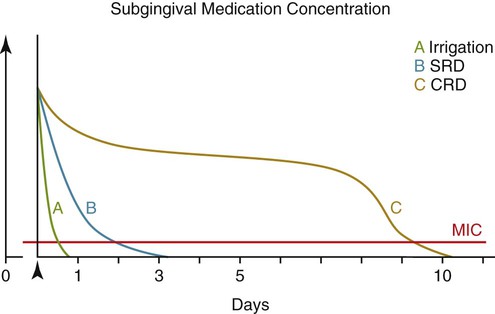
Chronic adult periodontitis is a multifactorial disease (Figure 89-4). The first requirement is a susceptible host, largely due to genetics and not modifiable. Another nonmodifiable risk factor is age. Other components of disease risk, which may be modifiable, include pathogenic oral plaque microorganisms and behavioral or environmental factors such as tobacco use. Mediating the pathogenesis are host-derived elements including immune and inflammatory responses that may impact soft and hard tissue metabolism and feed back upon the pathogenic flora. From the most basic perspective, however, periodontal diseases are bacterial infections; the requirement for bacteria to initiate the periodontal lesion is well recognized. Also well recognized are that (1) the antibacterial effect of SRP or other mechanical therapy generally is due to either a reduction of the bacterial load or an alteration of the composition of the bacterial flora at the periodontal site; and (2) the antibacterial effect of mechanical treatment alone is incomplete. Thus a good rationale exists to try to augment chemically the antibacterial effect of mechanical therapy. Multiple strategies have been used to try to deliver antimicrobial agents to the periodontal pocket at effective doses to impact the bacterial microflora including systemic administration or local administration via local irrigation of fluid formulations or placement of various gels or ointments. None of these strategies have proven to be as effective as controlled-release.
One of the drawbacks of antimicrobials delivered to the pocket but not in controlled-release formulations results from the dynamics of the GCF. The GCF fills the periodontal pocket space, but there is a copious flow of GCF out of the pocket that continuously moves GCF contents to the oral cavity.29,35 Additionally, the flow rate may be markedly enhanced in the presence of inflammation. Thus, antimicrobials delivered to the pocket are quickly washed out of the pocket by the GCF, rapidly reducing the concentration of drug locally to subantimicrobial levels (Figures 89-5 and 89-6). Drug concentrations within the GCF may even need to be elevated above usual antimicrobial levels because microorganisms within the pocket may exist within a protective biofilm structure in the periodontal ecosystem19,99 (for reviews, see Palmer75 or Kuboniwa and Lamont58). Bacterial biofilms may be highly resistant to penetration by fluids,26 providing further evidence for the critical need for high GCF concentrations of active antimicrobial, concentrations only achievable with suitable locally delivered controlled-release agents (see Pharmacokinetics, below) but not possible with locally delivered antimicrobials not in controlled-release formulations or via systemic routes. Additionally, Drisko24 has suggested that the high concentrations of antimicrobial in the GCF as a result of local delivery may help to reach infected sites within the root or tissue.
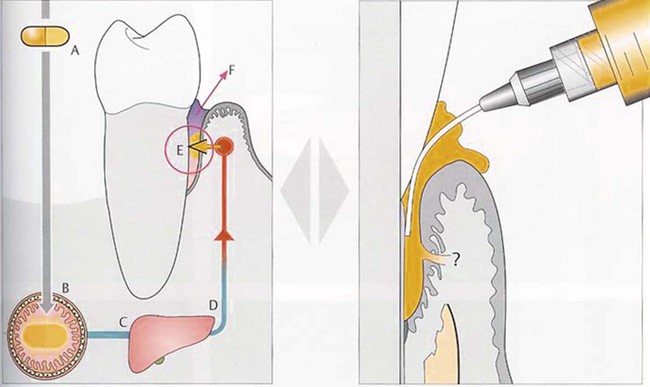
 , administration of locally delivered agent. (Adapted from Wolf HF, Hassell TM: Color atlas of dental hygiene: periodontology, ed 1, Stuttgart, Germany, 2006, Thieme, with permission.)
, administration of locally delivered agent. (Adapted from Wolf HF, Hassell TM: Color atlas of dental hygiene: periodontology, ed 1, Stuttgart, Germany, 2006, Thieme, with permission.)With regard to systemically delivered antimicrobials, drug concentrations within the GCF are orders of magnitude less than those achievable with controlled-release local delivery and cannot provide an equivalent, alternative therapy.92,103 Drug that is transferred from the plasma compartment to the GCF within the periodontal pocket is quickly washed away by the GCF flow (see Figure 89-5). It was previously thought that systemically delivered tetracyclines markedly concentrated drug within the GCF compared with plasma,33,79 but this hypothesis was not supported by later studies.92,103 A number of authors have suggested that systemic therapies, rather than locally delivered agents, should be considered when there are multiple pocket sites, both for convenience and to save costs,4,34,88 but it seems clear that if high concentrations of an antimicrobial drug are desired within the periodontal pocket, systemic delivery is not the appropriate therapy, regardless of the number of pocket sites. Other potential benefits of locally delivered, controlled-release antimicrobials may include decreased systemic, off-target effects or a decreased risk for promoting microbial resistance.
Pharmacokinetics
The pharmacokinetic principles of controlled-release local delivery are illustrated in Figure 89-6. Drug delivered locally to the periodontal pocket but not in a controlled-release formulation (e.g., via irrigation) is washed out of the pocket by the GCF flow, quickly reducing the concentration of drug within the GCF.29 Locally delivered, controlled-release antimicrobials have been designed to maintain high and clinically relevant concentrations of drug within the GCF for extended periods of time. The pharmacokinetics of the chlorhexidine chip, the doxycycline gel, and minocycline microspheres have been studied in clinical trials.
Chlorhexidine Chip.
The release profile of the chlorhexidine chip was evaluated in a single-center, 10-day clinical trial in 19 patients with chronic adult periodontitis.96 Each patient received a single chip administered into each of four pocket sites, and concentrations of chlorhexidine were determined in GCF, plasma, and urine at various times post dosing. Peak GCF concentrations were achieved 2 hours postdose (2007 ± 422 µg/mL), with GCF concentrations maintained between approximately 1300 and 1900 µg/mL to 4 days postdose. Mean chlorhexidine concentrations then steadily decreased to 57 ± 13 µg/mL at 9 days. Chlorhexidine was below detectable limits in plasma and urine at all time points. For perspective, Wilson et al108 reported that chlorhexidine, at a concentration of 125 µg/mL, killed 99.9% of cultivable bacteria from plaque samples from patients with chronic periodontitis within 15 minutes.
Doxycycline Gel.
The release profile of the doxycycline gel was evaluated in a single-center, 28-day clinical trial in 32 patients with adult periodontitis and compared with oral doxycycline.103 All patients assigned to receive local delivery had doxycycline gel administered to all pocket sites with probing depth 5 mm or more that bled on probing; drug was retained after placement either with a noneugenol (NE) periodontal dressing (n = 13) or 2-octyl cyanoacrylate (2-O; n = 13). Mean GCF concentrations peaked at 2 hours after dosing (1473 ± 328 µg/mL, NE; 1986 ± 445 µg/mL, 2-O). At day 7, mean concentrations were 309 ± 127 µg/mL (NE) and 148 ± 49 µg/mL (2-O). Mean salivary concentrations peaked at 2 hours (4.05 ± 1.24 µg/mL, NE; 8.78 ± 1.48 µg/mL, 2-O) and were ≤ 2 µg/mL at 24 hours. Serum concentrations following local administration remained ≤ 0.1 µg/mL. With regard to oral doxycycline (200 mg on day 0, then 100 mg/day for 7 days; n = 6), GCF concentrations peaked at 12 hours postdose (2.53 ± 1.56 µg/mL), and serum concentrations ranged from 0.91 to 2.26 µg/mL for the 8 days of data collection. Salivary concentrations never exceeded 0.11 µg/mL.
Minocycline Microspheres.
The release profile of minocycline microspheres was studied in 18 patients with moderate to advanced chronic periodontitis.6,7 Minocycline microspheres (mean dose: 46.2 mg; 25 to 112 unit doses) were administered to eligible periodontal pocket sites 5 mm or more in probing depth (minimum of 30 sites on at least eight teeth; 1 mg per treatment site) following SRP. Serum and saliva samples were collected predose and at various times postdose to 14 days. Mean dose-normalized saliva area under the curve and maximal concentration (Cmax) were approximately 125 and 1000 times that of serum parameters, respectively. In saliva minocycline, Cmax was 254.0 ± 139.3 mg/mL at 0.75 ± 0.56 hours postdose with a half-life of 44.7 ± 19.2 hours.7 In serum mean minocycline concentrations peaked at 4.8 ± 1.8 hours postdose (Cmax: 216.4 ± 122.9 µg/mL) and were undetectable by 7 days. Minocycline concentrations in saliva and GCF were sufficient (defined as >1 µg/mL) to kill bacteria associated with periodontal disease in vitro.7 These concentrations were evident up to 14 days without significant systemic exposure.7
Drug Development and Registration
An investigational drug is typically studied in many clinical trials before it can be approved for clinical use; locally delivered, controlled-release antimicrobials are no exception. These products have been rigorously studied in multiple trials to confirm their safety and efficacy for the indication of chronic adult periodontitis. This section includes a detailed discussion of the effectiveness of locally delivered, controlled-release antimicrobials as evidenced by trial data. A more detailed discussion of drug development and registration, including information regarding the phases of drug development, the evaluation of safety and efficacy, informed consent and trial design and information specifically relevant to periodontal studies, is provided in Chapter 90.
Locally Delivered, Controlled-Release Antimicrobials: Magnitude of the Effect
To consider results of clinical trials of locally delivered, controlled-release antimicrobials, a literature search for published trials was conducted in Ovid MEDLINE® from 1990 to 2012 using the search terms (1) chlorhexidine, minocycline, doxycycline, or tetracycline; (2) clinical trial, delayed action preparations/tetracycline/drug delivery systems/periodontal diseases/periodontitis/minocycline/local delivery, double-blind methods/placebo, parallel group, local drug delivery or pharmaceutical preparations/drug delivery systems/polymers/delayed-action preparations/drug carriers; and (3) periodontitis/chronic periodontitis, gingivitis/periodontal diseases/dental scaling/periodontal attachment loss/alveolar bone loss/periodontal pocket, planing, gingival crevicular fluid, periodontal index, periodontal treatment, or dental plaque. A total of 1216 articles were identified; of these, 186 were reviewed as potentially relevant. Overviews of results of reviewed trials of the doxycycline gel, minocycline microspheres, or the chlorhexidine chip used adjunctively or as monotherapy are presented in Tables 89-2, 89-3, and 89-4.
TABLE 89-2
Clinical Trials of Locally Delivered, Controlled-Release Antimicrobials (N ≥ 60 per treatment group)
| Drug | Study Design | Inclusion Criteria | Treatment Groups | Treatment | Study Duration | PD Outcomes (mean) | PD Reduction ≥2.0 mm from baseline | AL Outcomes (mean) | ||||
| Absolute Reduction from Baseline (mm) | Absolute Reduction Difference between Groups (mm) | Percent Change between Groups | Percent | Absolute Gain from Baseline (mm) | Absolute Gain Difference between Groups (mm) | Percent Change between Groups | ||||||
| DOX91 | Randomized, multicenter (10 ctrs), parallel-group, single-blind, monotherapy | Mod-sev periodontitis in ≥2 quads. Each quad had ≥4 pocket sites with PD ≥5 mm with BOP. Two sites had to be ≥ 7 mm. | DOX (1) Nonsmokers N = 52 (2) Former smokers N = 65 (3) current smokers N = 60 SRP (1) Nonsmokers N = 48 (2) Former smokers N = 72 (3) current smokers N = 61 |
All pts received OH. SRP pts rec’d SRP in the 2 treated quads, repeated at 4 mo (SRP until hard & smooth; local anesthesia PRN; no time restraints). DOX & veh at baseline and 4 mo applied to all qualifying pockets |
9 mo | DOX (1) 1.12 ± 0.11 (2) 1.33 ± 0.09 (3) 1.21 ± 0.10 SRP (1) 1.43 ± 0.11 (2) 1.05 ± 0.09 (3) 1.02 ± 0.10 |
(1) 0.31 P < 0.04 (2) 0.28, P <0.03 (3) 0.19 P = ns |
(1) 27% (2) 27% (3) 18% |
NR | DOX (1) 0.69 ± 0.12 (2) 0.88 ± 0.11 (3) 0.83 ± 0.11 SRP (1) 1.00 ± 0.12 (2) 0.60 ± 0.10 (3) 0.76 ± 0.11 |
(1) 0.31, P = ns (2) 0.28, P < 0.05 (3) 0.07 P = ns |
(1) 45% (2) 47% (3) 9% |
| DOX13 | Randomized, multicenter (3 ctrs), parallel-group, single-blind, adjunctive | ≥15 natural teeth, ≥4 w PD ≥ 5 mm, previously treated for moderate/ advanced chronic periodontitis, recall programs for SPT ≥ 1 y | (1) SPT alone, N = 65 (2) SPT + DOX, N = 63 |
SRP at baseline, no time limit, anesthesia if requested. In group (2), DOX in all sites w PD, PD ≥5 mm. 0.1% chlorhexidine rinse for 1 mo. Returned to clinic for SPT q 6 mo. At 1 & 2 y, DOX in sites w PD ≥5 mm | 3 y | (1) 1.1 (2) 1.2 [at 3 mo: (1) 0.7; (2) 0.9] |
0.1, P = ns [at 3 mo, 0.2, P < 0.001] |
9.1% [at 3 mo, 28.6%] |
NR | (1) 0.7 (2) 0.9 [at 3 mo: (1) 0.5; (2) 0.8] |
0.2, P = ns [at 3 mo, 0.3, P < 0.01] |
28.6% [at 3 mo, 60%] |
| TET25 | Randomized, multicenter (3 ctrs), split-mouth, single-blind, adjunctive and monotherapy | ≥1 site in each of 4 quads with PD ≥5 mm with BOP | (1) SRP alone (2) TET alone (3) TET alone administered twice, 10 d apart (4) SRP + TET N = 122 |
Supragingival scaling 2 wk before baseline. SRP w anesthesia for 5 min/tooth. Scaling at 10 d to remove any residual calc. | 12 mo, maintenance period | Sites <7 mm: 1.01–1.15 mm. Sites ≥7 mm: 1.63–2.39 mm |
0.17–0.29 mm. Some significant differences between groups at some time points, but not considered clinically significant | NR | NR | Sites <7 mm: 0.98–1.14 mm Sites ≥7 mm: 0.89–1.51 mm |
NR. No significant differences between groups | NR |
| TET68 | Multicenter (7 private practices) randomized, split-mouth, single-blind, adjunctive | Maintenance pts receiving regular SPT in private practice. Two nonadjacent sites in separate quadrants w PD 5–8 mm + BOP. | (1) SRP alone (2) SRP + TET fiber N = 113 |
Full-mouth SRP (no more SRP info). TET placed in test teeth. | 6 mo | (SD 6 mo) (1) 1.08 ± 0.121 (2) 1.81 ± 0.12 |
0.73 mm P < 0.01 |
68% | NR | (1) 1.08 ± 0.145 (2) 1.56 ± 0.145 |
0.48 P < 0.05 |
26% |
| TET30 | Multicenter (5 ctrs), randomized, split-mouth, single-blind, monotherapy | 4 nonadjacent teeth w PD 6–10 mm w BOP | N = 113 (1) TET (2) plc (3) SRP (4) No tx |
Prebaseline prophy; SRP for each tooth ≥5 min, local anesthesia. 10 d, fiber removal. |
60 d | (1) 1.05 ± 0.1 (2) 0.63 ± 0.1 (3) 0.74 ± 0.1 (4) 0.45 ± 0.09 |
1 vs 2: 0.42, P = 0.0001 1 vs 4 : 0.6, P = 0.0001 1 vs 3 : 0.31, P = 0.0002 2 vs 4 : 0.18, P = 0.2003 2 vs 3 : 0.11, P = 0.8711 3 vs 4 : 0.29, P = 0.1496 |
1 vs 2: 67% 1 vs 4 : 133% 1 vs 3 : 42% 2 vs 4 : 15% 2 vs 3 : 17% 3 vs 4 : 64% |
NR | (1) 0.66 ± 0.13 (2) 0.49 ± 0.12 (3) 0.41 ± 0.12 (4) 0.37 ± 0.12 |
1 vs 2: 0.17, P = 0.0456 1 vs 4 : 0.29, 0.0150 1 vs 3 : 0.25, P = 0.0111 2 vs 4 : 0.12, 0.6606 2 vs 3 : 0.08, P = 0.5795 3 vs 4 : 0.04, P = 0.9072 |
1 vs 2: 35% 1 vs 4 : 78% 1 vs 3 : 61% 2 vs 4 : 32% 2 vs 3 : 20% 3 vs 4 : 11% |
| MIN107 | Parallel-group; double-blind; adjunctive therapy; multicenter (18 ctrs) | ADA Type III (mod) or Type IV (sev) periodontitis. ≥4 teeth w PD 6–9 mm w BOP | (1) SRP alone, N = 250; (2) SRP + vehicle, N = 249; (3) SRP + MIN, N = 249; All sites ≥5 mm received study drug or vehicle at baseline, 3 & 6 mo |
Full-mouth SRP at baseline; No limits: time or local anesthesia | 9 mo | 9 mo: (1) 1.08 (0.04) mm (2) 1.00 (0.04) (3) 1.32 (0.04) |
1 vs 2: 0.08, P = ns 1 vs 3: 0.24, P < 0.001 2 vs 3: 0.32, P < 0.001 |
22% greater reduction vs SRP alone 32% greater reduction vs SRP + vehicle (both p < 0.001) |
(1) 32.87%; (2) 28.98%; (3) 40.02%* *Diff from other 2 groups p < 0.001 |
NR | NA | NA |
| CHX76 | Split-mouth; single-blind; adjunctive therapy; multicenter (4 ctrs) | Two sites per pt; PD ≥5 mm; BOP | (1) SRP alone (1 site); (2) SRP + CHX chip (1 site) N = 116 |
Two 2-h sessions within 48 h; w local anesthesia; full-mouth subgingival prophy at 3 mo | 6 mo | Est from figure 3 mo (1) 1.2 (2) 1.5 6 mo (1) 1.0 (2) 1.5 |
0.30 mm (p < 0.01) at 3 mo; 0.55 mm (p < 0.001) at 6 mo vs SRP alone | 113% increase vs SRP alone (p < 0.01) at 6 mo | NR | Est from figure 3 mo (1) 0.6 (2) 0.9 6 mo (1) 0.5 (2) 1.1 |
0.28 mm at 3 mo; 0.64 mm at 6 mo vs SRP alone | 128.6% vs SRP alone |
| CHX97 | Randomized, single-blind, multicenter (3 ctrs), adjunctive therapy, split-mouth | ≥1 pocket, PD 5–8 mm, w BOP, in each max quadrant | (1) SRP alone; N = 118 pts; 485 pockets (2) SRP + CHX chip N = 118 pts; 474 pockets |
Full mouth SRP (tx time limited to 1 h). At 3 mo, full mouth supragingival prophy per clin need. CHX inserted if PD 5–8 mm | 6 mo | (1) 0.70 ± 0.056 (2) 1.16 ± 0.058 |
0.46 p ≤ 0.0001 |
65.7% | (1) 21.3% ± 2.0 (2) 35.4% ± 2.1 both p ≤ 0.0001 |
(1) 0.31 ± 0.06 (2) 0.47 ± 0.06 |
0.16 P < 0.05 |
51.6% |
| CHX49 | 2 Multicenter, randomized, double-blind, parallel-group, adjunctive therapy (5 ctrs each) | ≥10 teeth, ≥4 teeth w PD ≥5 mm, BOP. Stratified by smoking status | Group 1 pts (active chip) SRP + CHX chip (Tx 1). SRP alone, N = 225 Group 2 pts (plcb chip) SRP + plcb (Tx 2); SRP alone (Tx 3). N = 222 |
Supragingival prophy for 1 h. SRP for 1 h for all teeth. Chips in test sites at baseline, at 3 & 6 mo if PD still ≥5 mm. Exit prophy at 9 mo | 9 mo | (1) 0.95 ± 0.05 (2) 0.69 ± 0.05 (3) 0.65 ± 0.05 |
1–3 0.26 ± 0.08, P = 0.00056 1–2 0.30 ± 0.07 P = 0.00001 |
46% greater reduction vs SRP alone (p = 0.00001) 38% greater reduction vs SRP + vehicle (p = 0.00056) |
139% increase vs SRP alone (p < 0.0001) 48% increase vs SRP + vehicle (p = 0.039) |
(1) 0.75 ± 0.06 (2) 0.55 ± 0.06 (3) 0.58 ± 0.06 |
1–3 0.16 ± 0.08 P = 0.05 1–2 0.20 ± 0.068 P = 0.012 |
29% gain vs SRP alone; 34% gain vs SRP + vehicle |
| DOX27 Study 1 |
Randomized, multicenter (10 ctrs), parallel-group, single-blind, monotherapy | Mod-sev periodontitis in ≥2 quads. Each quad had ≥4 pocket sites with PD ≥5 mm with BOP. Two sites had to be ≥7 mm. | (1) DOX, N = 95; (2) Veh N = 94 (3) OH N = 95 (4) SRP N = 99 |
All pts received OH. SRP pts rec’d SRP in the 2 treated quads, repeated at 4 mo (SRP until hard & smooth; local anesthesia PRN; no time restraints). DOX & veh at baseline and 4 mo applied to all qualifying pockets |
9 mo | (1) 1.1 ± 0.1 (2) 0.8 ± 0.1 (3) 0.5 ± 0.1 (4) 0.9 ± 0.1 |
1 vs 2, 0.3, p = 0.001; 1 vs 3, 0.6, p < 0.001; 1 vs 4, 0.2, p = 0.050 |
1 vs 2: 37.5% 1 vs 3: 120% 1 vs 4: 22.2% |
NR | (1) 0.8 ± 0.1 (2) 0.1 ± 0.1 (3) 0.3 ± 0.1 (4) 0.7 ± 0.1 |
1 vs 2, 0.7, p < 0.001 1 vs 3, 0.5, p = 0.001 1 vs 4, 0.1, p = 0.294 |
1 vs 2: 700% 1 vs 3: 166% 1 vs 4: 14.3% |
| DOX27 Study 2 |
Randomized, multicenter (10 ctrs), parallel-group, single-blind, monotherapy | Mod-sev periodontitis in ≥2 quads. Each quad had ≥4 pocket sites with PD ≥ 5 mm with BOP. Two sites had to be ≥7 mm. | (1) DOX, N = 95; (2) Veh N = 94 (3) OH N = 95 (4) SRP N = 99 |
All pts received OH. SRP pts rec’d SRP in the 2 treated quads, repeated at 4 mo (SRP until hard & smooth; local anesthesia PRN; no time restraints). DOX & Veh at baseline and 4 mo applied to all qualifying pockets |
9 mo | (1) 1.3 ± 0.1 (2) 1.0 ± 0.1 (3) 0.9 ± 0.1 (4) 1.3 ± 0.1 |
1 vs 2, 0.3, p = 0.001; 1 vs 3, 0.4, p < 0.001; 1 vs 4, 0.0, p = 0.765 |
1 vs 2: 30% 1 vs 3: 44.4% 1 vs 4: 0% |
NR | (1) 0.8 ± 0.1 (2) 0.5 ± 0.1 (3) 0.5 ± 0.1 (4) 0.9 ± 0.1 |
1 vs 2, 0.3, p = 0.002 1 vs 3, 0.3, p = 0.012 1 vs 4, 0.1, p = 0.665 |
1 vs 2: 60% 1 vs 3: 60% 1 vs 4: 12.5% |
| CHX98 | Open-label, 8 private dental offices, Phase IV | ≥30 y age; pockets w PD ≥5 mm; no perio sx or SRP by periodontist within 2 y of entrance | SRP group Non-SRP group (already on active RPMT) N = 835 enrolled; 595 completed at 24 mo |
SRP (SRP group only) 1 mo before baseline. RPMT q 3 mo. All sites receiving CHX chip at baseline got CHX q 3 mo if PD ≥5 mm | 2 y | 12 mo, 4.98 ± 1.01 24 mo, 4.77 ± 1.05 |
NA | NA | ≥1 site 12 mo, 42.88 24 mo, 51.26 ≥2 sites 12 mo, 17.93 24 mo, 23.19 |
|||
| MIN31,12 | Multicenter, single-blind, randomized, parallel-group, Phase IV | 30-65 y age; ≥16 teeth; 5 sites w PD ≥5 mm in 5 nonadjacent interproximal spaces (excluding distal of terminal teeth; no perio tx or antibiotics within 3 mo | (1) SRP alone, N = 65 (2) SRP + MIN, N = 62 |
Full-mouth SRP in ≤2 visits ≤10 d apart w hand, ultrasonic instruments, & local anesthesia permitted. MIN administered for group 2 to sites w PD ≥5 mm | ||||||||
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


