Chapter 29
Cements Based on Organometallic Chelate Compounds
29.1 Introduction
Many cements used in dentistry can be characterised by setting reactions which involve the formation of chelate compounds between divalent metallic ions such as zinc ions and ortho-disubstituted aromatic compounds. Three types of aromatic compound are commonly used, delineating the three groups of materials.
29.2 Zinc oxide/eugenol cements
Composition and setting: These products may be supplied as a powder and liquid or as two pastes (Figs. 29.1 and 29.2). The composition of a typical powder/liquid material is given in Table 29.1. The small quantity of zinc acetate in the powder acts as an accelerator by helping to create an ionic medium in which the setting reaction can occur. Some commercial products contain hydrogenated rosin or polystyrene and are known as resin-reinforced zinc oxide/eugenol cements. The relative proportions of powder and liquid are not normally measured accurately, although some manufacturers provide a scoop which gives a known volume of powder to which a given number of drops of liquid are added. Thin mixes, having a low powder/liquid ratio, should be avoided since they produce inferior properties – lower strength and higher solubility. The paste/paste materials are similar to the impression pastes described on p. 151. These have the advantage of easier proportioning and mixing.
The setting reaction involves chelation of two eugenol molecules (see Fig. 17.5) with one zinc ion to form zinc eugenolate (see Fig. 17.6). This reaction proceeds very slowly in the absence of moisture. When the mixed material contacts water, however, setting is often completed within a few seconds.
Properties: The setting characteristics of the zinc oxide/eugenol cements are, to some extent, ideal. They offer a combination of adequate working time, during which very little increase in viscosity occurs, coupled with rapid setting after placing into the cavity. The latter is caused by residual moisture in the cavity and the higher temperature of the mouth compared with room temperature. The effect of cavity moisture is noteworthy, particularly since efforts are generally made to dry the cavity before placement of a lining. Only very small amounts of water are required to cause the accelerating effect.
The ultimate compressive strength values for the zinc oxide/eugenol cements are somewhat lower than those recorded for zinc phosphate materials, typically 20 MPa for unreinforced materials and 40 MPa for reinforced materials, The nature of the setting reaction is such, however, that the materials develop their strength rapidly and the reinforced materials in particular are unlikely to flow or fracture during amalgam condensation, providing correct technique is used.
Zinc oxide/eugenol cements may be used as linings in deep cavities without causing harm to the pulp. Unconsumed eugenol is able to leach from the set material and although this substance has been shown to be irritant under certain conditions, it appears to have an obtundant effect on the pulp. The free eugenol is also bacteriocidal which helps to minimize the effects of bacterial ingress and the production of exotoxins causing pulpal damage as a consequence of microleakage.
Fig. 29.1 Zinc oxide eugenol cavity base and cavity lining materials. The materials consists of a white powder which is primarily zinc oxide. The liquid contains mainly eugenol, which is derived from oil of cloves. The smell of eugenol is one of the traditional odours of the dental surgery. The powder and liquid are mixed together before undergoing a setting reaction.
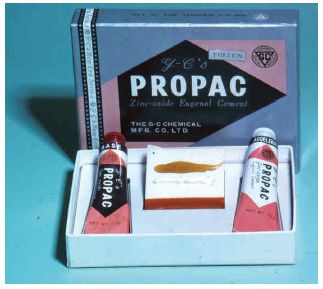
Fig. 29.2 A zinc oxide eugenol cement provided in the form of two pastes. The active ingredients are very similar to those in the powder/liquid system shown in Fig. 29.1. Two pastes are mixed together on a mixing pad.
The ease with which eugenol can gain egress from the material is responsible for its relatively high solubility. Leached eugenol is replaced by water which, under certain conditions, can cause hydrolysis of the zinc eugenolate and disintegration of the cement structure. The materials are, therefore, not suitable for luting applications except on a temporary basis.
Table 29.1 Composition of zinc oxide/eugenol cements.
| Component | Function | |
| Powder | Zinc oxide | Primary reactive ingredient |
| Zinc acetate (1–5%) | Accelerator | |
| Liquid | Eugenol | Primary reactive ingredient |
| Olive oil (5–15%) | To control viscosity |
Fig. 29.3 A zinc oxide eugenol paste used in a temporary cement.
Free eugenol may also have an effect on resinbased filling materials, interfering with the polymerisation process and sometimes causing discoloration. Clinically this can also result in softening of the surface of the composite. In addition there can be problems with resin-based adhesive luting systems that are being used increasingly, if a provisional restoration is luted using a zinc oxide eugenol temporary cement.
The materials form an effective thermal barrier under metallic restorations having a value of thermal diffusivity similar to that for dentine.
The main uses of these cements are for linings under amalgam restorations, either used alone or as a sublining overlaid with a zinc phosphate material. They are also used as temporary luting cements and as temporary filling ma/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


