Chapter 2
Comparison of periodontal debridement instrumentation modalities
CHAPTER OBJECTIVES
- To review the available methods of periodontal debridement including the use of manual instrumentation, ultrasonic instrumentation, and laser energy.
- To review the evidence that compares the potential for manual and ultrasonic instrumentation to remove calculus and biofilm.
- To review the evidence that compares the potential for manual and ultrasonic instrumentation to reduce probing depth, increase attachment level, and reduce bleeding on probing (BOP).
- To assess the difference between manual and ultrasonic instrumentation in terms of root surface damage during use.
- To review the evidence concerning the use of ultrasonic instrumentation during debridement of dental implant surfaces.
We learned in the first chapter that root planing is an outdated concept. In this new age of root surface debridement, the goal of periodontal therapy is biofilm disruption and preservation of root cementum. Biofilm disruption can be accomplished by mechanical means (manual instrumentation and/or ultrasonic instrumentation), and laser-generated energy (Rosen, 2001). With the objectives of modern periodontal debridement clearly in mind (Box 1), attention should next be focused on the efficacy, advantages, and limitations of available instrumentation techniques to achieve these objectives.
EFFICACY OF DEPOSIT REMOVAL
As explained in Chapter 1, it is the formation of bacteria into complex biofilms that initiates the host response that results in periodontal breakdown, with the presence of calculus deposits contributing to the retention of plaque. Visible plaque forms when biofilm grows to the point where it is clinically detectable. We also think of calculus in terms of calcified deposits that can be seen or felt. But, the fact is that not all biofilm develops into plaque, not all plaque forms into calculus, and not all of the calculus that forms is clinically detectable.
Box 2.1 Objectives of modern periodontal debridement
- Disruption and removal of the subgingival biofilm
- Removal of plaque retentive factors such as calculus
- Conservation of tooth structure
- Creation of a biologically acceptable root surface
- Resolution of inflammation
For the resolution of periodontal disease, the subgingival microbiota and plaque-retentive deposits must be reduced to a level compatible with periodontal health. Admittedly, the level (of reduction) necessary to facilitate disease resolution is likely to differ between patients, as it is dependent on the host immune system and variations in the presence or absence of risk factors associated with periodontal diseases. Therefore, rather than aiming for an unattainable objective of complete removal of all deposits, an instrumentation method that can predictably remove as much clinically evident and microscopic etiology as possible should be used to insure that an adequate reduction is achieved in all individuals.
Calculus removal
While a number of studies confirm that nonsurgical instrumentation is effective in significantly reducing the amount of calculus on root surfaces, complete calculus removal is not usually achieved (Chapter 1). Comparisons between studies assessing the ability for manual or ultrasonic instrumentation to remove calculus are difficult to make owing to the inability to standardize the characteristics of deposit being removed, the methodology used to remove it, or even the detection of calculus itself before or after therapy. With these limitations in mind, it is not surprising that there is no complete agreement on the calculus removal abilities of different methods of instrumentation (Cobb, 1996). However, most studies suggest that ultrasonic instrumentation removes calculus to a degree similar to that removed via manual instrumentation (Hunter et al., 1984; Breininger et al., 1987; Kepic et al., 1990; Thornton and Garnick, 1992; Apatzidou and Kinane, 2010). One such study of 60 teeth slated for extraction was undertaken in part to identify and quantify the residual deposits on instrumented root surfaces. Two molar and two nonmolar tooth groups (of 15 teeth each) were alternately treated in vivo by manual instrumentation either with a variety of curettes or an ultrasonic instrument. Instrumented and control teeth were then extracted, stained, and analyzed via stereomicroscopy using a calibrated grid. Both manual and ultrasonic instrumentation as performed in this study were equally effective in calculus removal. It is noteworthy that in both treatment groups, the remaining calculus was generally found to be free of live bacteria. Thus, while neither method resulted in complete calculus removal, both methods were remarkably effective in bacterial debridement of subgingival root surfaces (Breininger et al., 1987).
More recently, 12 extracted mandibular incisors, each with similar levels of clinical root calculus were instrumented by either a manual curette or an ultrasonic tip until no visible calculus remained. Photomicrographs obtained via scanning electron microscopy were used to determine and compare levels of remaining calculus following each method of instrumentation. Assessment of the residual calculus showed no difference between the root surfaces treated by manual or ultrasonic instrumentation (Marda et al., 2012).
Thus, it seems reasonable to conclude that ultrasonic and manual instrumentation have equal capability to remove calculus. Neither method resulted in complete calculus removal. However, as reviewed in the first chapter and further detailed in the next section, both methods removed sufficient calculus during use to result in resolution of periodontal disease.
Biofilm removal
Early studies confirm the ability for both manual and ultrasonic instrumentation to have equivalent beneficial effects on the periodontal etiology (Thornton and Garnick, 1982; Oosterwaal et al., 1987; Renvert et al., 1990), with ultrasonic instrumentation possibly offering an advantage in bacterial removal in furcation areas (Leon and Vogel, 1987).
Laboratory methods for bacterial identification have been refined considerably compared to the methods that were available at the time of these early studies. In a more recent study, DNA hybridization was employed to assess microbiological changes following manual and ultrasonic instrumentation (Iannou et al., 2009). Changes in levels of the so-called red complex pathogens that play a key role in periodontal disease development (Socransky et al., 1998) were assessed over a 6-month period. Teeth were treated either via manual instrumentation using a variety of curettes or by ultrasonic instrumentation using a piezoelectric device. In both cases, instrumentation continued until a “smooth, hard” surface was achieved as assessed clinically by using an explorer.
In alignment with the results of earlier studies, microbiological samples obtained at baseline and at 3 and 6 months demonstrate that manual and ultrasonic instrumentation are comparable in their ability to alter the periodontal bacteria (Table 2.1).
Table 2.1 Microbial changes at 3 and 6 months after root debridement via manual (MI) and ultrasonic (USD) instrumentation
| Mean numbers (x 105, mean ± SEM) of the four species tested | ||||
| Species/Instrumentation method | Baseline | 3 months | 6 months | |
| Pg | MI | 7.36 ± 2.29 | 2.63 ± 0.721 | 1.39 ± 0.441 |
| USD | 5.45 ± 0.99 | 2.76 ± 0.691 | 2.45 ± 0.591 | |
| Aa | MI | 1.51 ± 0.43 | 2.21 ± 0.53 | 0.99 ± 0.19 |
| USD | 1.745 ± 0.69 | 1.94 ± 0.71 | 1.66 ± 0.49 | |
| Tf | MI | 4.20 ± 1.57 | 0.75 ± 0.28 | 0.83 ± 0.24 |
| USD | 3.06 ± 0.73 | 1.29 ± 0.49 | 2.44 ± 0.611 | |
| Td | MI | 3.03 ± 1.54 | 0.88 ± 0.23 | 0.74 ± 0.38 |
| USD | 2.64 ± 0.073 | 1.05 ± 0.41 | 3.18 ± 1.21 | |
Pg, Porphyromonas gingivalis; Aa, Aggregatibacter actinomycetemcomitans; Tf, Tannerella forsythia; Td, Treponema denticola.
1 Statistically significant difference from baseline (Wilcoxon’s Signed Ranks test, p < 0.05).
Source: Data from Ioannou et al., 2009.
To optimize the mechanical disruption of biofilm, particularly at the microscopic level, maximum contact between the tooth surface and the working end of the tip is required, regardless of the type of instrument used. It is here, relative to biofilm disruption, that the mechanisms of action and tip design inherent to ultrasonic instrumentation provide distinct advantages over manual instrumentation.
Manual instruments were designed with a bladed edge (Figure 2.1) primarily to cut calcified deposits and plane contaminated cementum from the tooth surface, with the resulting plaque removal being incidental. Using a bladed instrument at an appropriate force to remove the biofilm without cementum removal, while difficult in itself, requires constant effort to adequately adapt the straight cutting edge to the curvatures of the tooth and only results in biofilm interruption at the point of contact. To achieve maximum contact, the cutting edge of the instrument must be honed throughout the procedure. The integrity of the cutting edge is altered with contact between the blade and tooth/deposit/restoration surfaces. When altered, at the ultrastructural level, gaps where no contact is made between the edge and the root surface result, essentially leaving some portions of the root receiving only minimal debridement.
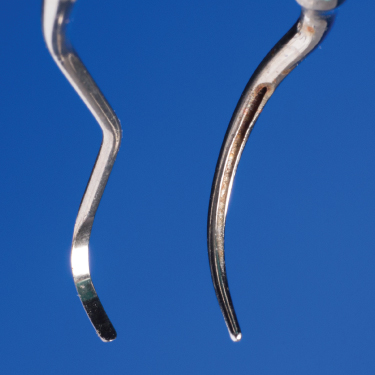
Figure 2.1 Comparison of the bladed, linear working end of a curette to the blunt, cylindrical active area of an ultrasonic tip
Conversely, the cylindrical shape of the blunt ultrasonic tip (Figure 2.1) is more conducive to biofilm removal than the linear cutting edge of a manual instrument, as it naturally conforms to the curvatures of the tooth surface (Chapter 4), and the biophysical forces of cavitation and microstreaming extend the interruption of biofilm beyond the point of direct tip contact (Chapter 3).
The advantages of ultrasonic instrumentation as described above are never more evident than during the debridement of furcations. There is general agreement among clinicians that effective instrumentation is considerably more difficult to achieve in areas of furcation involvement. It is of interest to note that studies in root morphology have shown that the measured entrance to furcation areas is often less than the blade width of curettes (Hou et al., 1994). This can severely limit the accessibility of furcations to thorough debridement with manual instrumentation.
In a study comparing manual and ultrasonic debridement of 33 molar furcations in 6 adult patients, ultrasonic and manual instrumentation were equally effective in reducing microbial counts (via dark field microscopy) in class I furcations. However, ultrasonic instrumentation was clearly superior in decreasing inflammation and motile forms of bacteria in class II and class III furcations (Leon and Vogel, 1987). Hence, ultrasonic devices may be the instruments of choice for debriding furcation and other areas with challenging root morphologies (Patterson et al., 1989; Oda and Ishikawa, 1989; Takacs et al., 1993).
In summary, although studies demonstrate that both manual and ultrasonic instrumentation sufficiently interrupt biofilm, the improved conformity afforded by the design of ultrasonic tips combined with the mechanisms of ultrasonic debridement (vibratory and biophysical) increases the likelihood that the biofilm will be disrupted below the threshold necessary to result in resolution of disease.
RESOLUTION OF DISEASE
The typical parameters that are assessed to determine the presence or progression of periodontal disease include probing depth, clinical attachment level (CAL), and inflammation (usually measured by BOP). The effect that ultrasonic or hand instrumentation has on these common parameters has been widely studied. While it is admittedly difficult to standardize all variables (e.g., operator skill level, time allotted per tooth or per arch, specific instrument type, type of deposits, morphology of involved roots), some generalizations can be made to determine the usefulness of the various debridement modalities. With the data demonstrating comparable efficacy of manual and ultrasonic instrumentation to remove root deposits in mind, it is reasonable to expect both methods of instrumentation to result in similar levels of clinical improvement.
Probing depth
A systematic review of 27 studies indicated that mean probing depth reductions following hand instrumentation ranged from 1.29 mm for pockets with moderate probing depth to 2.16 mm for pockets with deep probing depths (Cobb, 1996). Studies that assessed probing depth reduction following the use of ultrasonic and sonic instruments yielded results almost identical to those achieved by manual instrumentation (Torfason et al., 1979; Badersten et al., 1981, 1984; Copulos et al., 1993; Boretti, 1995; Kocher, 2001, Ioannou et al., 2009) (Table 2.2a).
Table 2.2a Mean changes in probing depth (PD) resulting from manual (MI) and ultrasonic (UI) instrumentation
| Study | Method | Reduction in PD (mm) |
| Torfason et al., 1979 | MI | 1.70 |
| UI | 1.72 | |
| Badersten et al., 1981 | MI | 1.0 |
| UI | 1.2 | |
| Badersten et al., 1984 | MI | 1.4 |
| UI | 1.2 | |
| Copulos et al., 1993 | MI | 0.72 |
| UI | 0.75 | |
| Boretti et al., 1995 | MI | 1.83 |
| UI | 1.82 | |
| Kocher et al., 2001 | MI | 0.77 |
| UI | 1.10 | |
| Ioannou et al., 2009 | MI | 0.88 |
| UI | 0.44 |
There is little doubt that a reduction in the pocket depth improves access to subgingival areas for the therapist and patient alike. Root surfaces adjacent to shallow pockets are more easily accessed by clinical instrumentation during debridement and by the patient during daily hygiene efforts. Therefore, reduction of probing depth is a laudable treatment goal as it reduces the likelihood of relapse. However, focusing the management of periodontal disease solely on pocket depth may not be sufficient.
Attachment level
Compared to probing depth, CAL is the more accurate indicator of the amount of periodontal tissue destruction that has taken place (Figure 2.2). Posttreatment, CAL allows the examiner to determine if a positive change in probing depth is due to a gain in periodontal attachment or simply due to recession of the soft tissues.
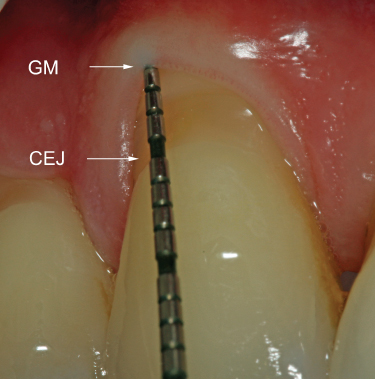
Figure 2.2 Probe indicates PD = 1 mm, with 4 mm of recession; thus, CAL = 5 mm. GM: gingival margin; CEJ: cementoenamel junction
As with probing depth, gains in CAL are comparable between ultrasonic/sonic and manual debridement in the treatment of periodontitis (Badersten et al., 1981, 1984; Copulos et al., 1993; Boretti, 1995; Kocher, 2001; Ioannou, 2009) (Table 2.3). Thus, both manual and ultrasonic instrumentation have the potential to not only reduce pocket depth but also to result in gains in clinical attachment.
Table 2.2b Mean changes in disease parameters resulting from manual (MI) and ultrasonic (UI) instrumentation
| Study | Method | Reduction in PD (mm) | Gain in CAL (mm) | Reduction in % of sites with BOP |
| Torfason et al., 1979 | MI | 1.70 | 45% | |
| UI | 1.72 | 45% | ||
| Badersten et al., 1981 | MI | 1.0 | 1.39 ± 0.44 | 64% |
| UI | 1.2 | 2.45 ± 0.59 | 63% | |
| Badersten et al., 1984 | MI | 1.4 | 0.99 ± 0.19 | 51% |
| UI | 1.2 | 1.66 ± 0.49 | 52% | |
| Copulos et al., 1993 | MI | 0.72 | 0.74 ± 0.38 | |
| UI | 0.75 | 3.18 ± 1.21 | ||
| Boretti et al., 1995 | MI | 1.83 | 1.53 | 91% |
| UI | 1.82 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


