Desquamative Gingivitis
Alfredo Aguirre, Jose Luis Tapia Vazquez and Russell J. Nisengard†
Chronic Desquamative Gingivitis
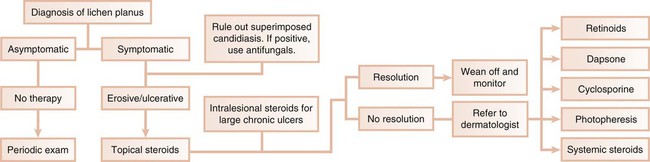
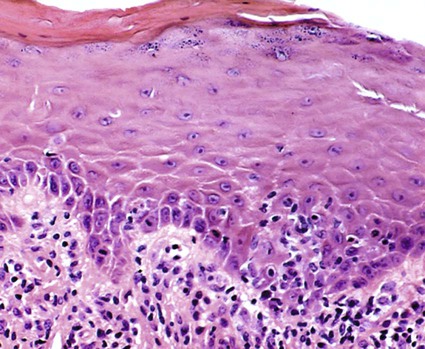
Although first recognized and reported in 1894,157 the term chronic desquamative gingivitis was coined in 1932 by Prinz122 to describe a peculiar condition characterized by intense erythema, desquamation, and ulceration of the free and attached gingiva50,100 (Figure 19-1). Patients may be asymptomatic; when symptomatic, however, their complaints range from a mild burning sensation to an intense pain. Approximately 50% of desquamative gingivitis cases are localized to the gingiva, although patients can have involvement of the gingiva plus other intraoral and even extraoral sites.56,112 Initially, the cause of this condition was unclear, with a variety of possibilities suggested. Because most cases were diagnosed in women during the fourth and fifth decades of life (although desquamative gingivitis may occur as early as puberty or as late as the seventh or eighth decade), a hormonal derangement was suspected. In 1960, however, McCarthy and colleagues94 suggested that desquamative gingivitis was not a specific disease entity but rather a gingival response associated with a variety of conditions. This concept has been further supported by numerous immunopathologic studies.77,114,130,151
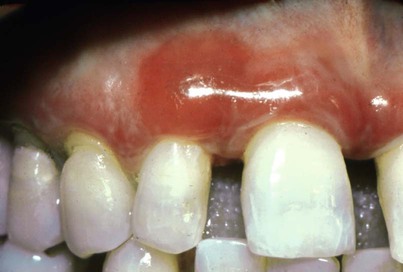
The use of clinical and laboratory parameters has revealed that approximately 75% of desquamative gingivitis cases have a dermatologic genesis. Cicatricial pemphigoid and lichen planus account for more than 95% of the dermatologic cases.110 However, many other mucocutaneous autoimmune conditions (e.g., bullous pemphigoid, pemphigus vulgaris, linear immunoglobulin A [IgA] disease, dermatitis herpetiformis, lupus erythematosus, chronic ulcerative stomatitis) can clinically manifest as desquamative gingivitis.138
Other conditions that must be considered in the differential diagnosis of desquamative gingivitis include chronic bacterial, fungal, and viral infections as well as reactions to medications, mouthwashes, and chewing gum. Although less common, Crohn’s disease, sarcoidosis, some leukemias, and even factitious lesions have also been reported to present clinically as desquamative gingivitis.138,161
Therefore, it is of paramount importance to ascertain the identity of the disease responsible for desquamative gingivitis to establish the appropriate therapeutic approach and management. To achieve this goal, the clinical examination must be coupled with a thorough history and routine histologic and immunofluorescence studies.154,161 Despite this diagnostic approach, however, the cause of desquamative gingivitis cannot be elucidated in up to one third of the cases.125
Diagnosis of Desquamative Gingivitis: A Systematic Approach
Desquamative gingivitis is only a clinical term and not a diagnosis per se. Once the presence of this condition is determined, a series of laboratory procedures should be used to arrive at a final diagnosis. Thus, the success of any given therapeutic approach depends on the establishment of an accurate final diagnosis. The following discussion represents a systematic approach to elucidate the disease that is triggering desquamative gingivitis (Figure 19-2).
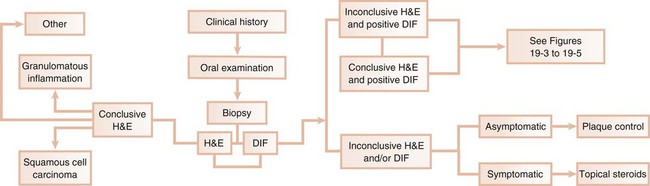
Clinical History
A thorough clinical history is mandatory to begin the assessment of desquamative gingivitis.113 Data regarding the symptomatology associated with this condition as well as its historical aspects (i.e., when the lesion started, whether it has worsened, if there is a habit that exacerbates the condition) provide the foundation for a thorough examination. Information regarding previous therapy to alleviate the condition should also be documented.
Clinical Examination
Recognition of the pattern of distribution of the lesions (i.e., focal or multifocal, with or without confinement to the gingival tissues) provides leading information to begin the formulation of a differential diagnosis. In addition, a simple clinical maneuver such as Nikolsky’s sign offers insight into the plausibility of the presence of a vesiculobullous disorder.97
Biopsy
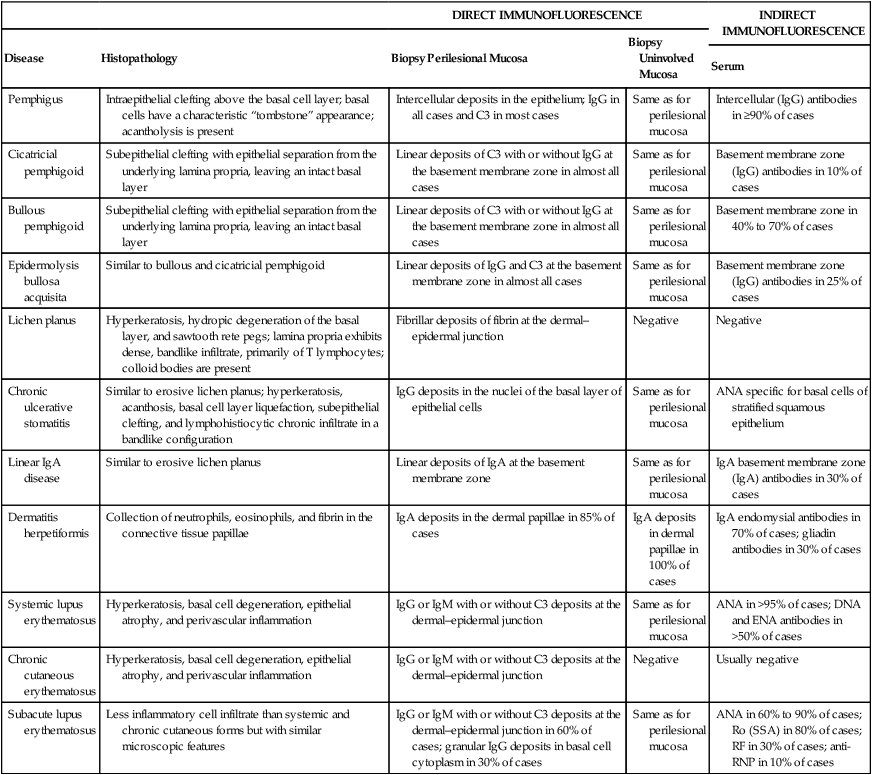
Given the extent and number of lesions that may be present in an individual, an incisional biopsy is the best alternative to use to begin the microscopic and immunologic evaluation. An important consideration is the selection of the biopsy site. A perilesional incisional biopsy should avoid areas of ulceration, because necrosis and epithelial denudation severely hamper the diagnostic process. After the tissue is excised from the oral cavity, the specimen can be bisected and then submitted for microscopic examination. Buffered formalin (10%) should be used to fix the tissue for conventional hematoxylin-eosin (H&E) evaluation. Michel’s buffer (ammonium sulfate buffer, pH 7.0) is used as transport solution for immunofluorescence assessment. In general, an incisional biopsy of uninvolved (normal) mucosa will show the same immunofluorescent findings as the biopsy of the perilesional tissue. However, there are notable exceptions, such as lichen planus and chronic cutaneous lupus erythematosus, in which only the lesional tissue will exhibit the corresponding immunologic markers (Table 19-1).
TABLE 19-1
Histopathologic, Direct, and Indirect Immunofluorescence Findings in Select Conditions that may Present Clinically as Desquamative Gingivitis
| DIRECT IMMUNOFLUORESCENCE | INDIRECT IMMUNOFLUORESCENCE | |||
| Disease | Histopathology | Biopsy Perilesional Mucosa | Biopsy Uninvolved Mucosa | |
| Serum | ||||
| Pemphigus | Intraepithelial clefting above the basal cell layer; basal cells have a characteristic “tombstone” appearance; acantholysis is present | Intercellular deposits in the epithelium; IgG in all cases and C3 in most cases | Same as for perilesional mucosa | Intercellular (IgG) antibodies in ≥90% of cases |
| Cicatricial pemphigoid | Subepithelial clefting with epithelial separation from the underlying lamina propria, leaving an intact basal layer | Linear deposits of C3 with or without IgG at the basement membrane zone in almost all cases | Same as for perilesional mucosa | Basement membrane zone (IgG) antibodies in 10% of cases |
| Bullous pemphigoid | Subepithelial clefting with epithelial separation from the underlying lamina propria, leaving an intact basal layer | Linear deposits of C3 with or without IgG at the basement membrane zone in almost all cases | Same as for perilesional mucosa | Basement membrane zone in 40% to 70% of cases |
| Epidermolysis bullosa acquisita | Similar to bullous and cicatricial pemphigoid | Linear deposits of IgG and C3 at the basement membrane zone in almost all cases | Same as for perilesional mucosa | Basement membrane zone (IgG) antibodies in 25% of cases |
| Lichen planus | Hyperkeratosis, hydropic degeneration of the basal layer, and sawtooth rete pegs; lamina propria exhibits dense, bandlike infiltrate, primarily of T lymphocytes; colloid bodies are present | Fibrillar deposits of fibrin at the dermal–epidermal junction | Negative | Negative |
| Chronic ulcerative stomatitis | Similar to erosive lichen planus; hyperkeratosis, acanthosis, basal cell layer liquefaction, subepithelial clefting, and lymphohistiocytic chronic infiltrate in a bandlike configuration | IgG deposits in the nuclei of the basal layer of epithelial cells | Same as for perilesional mucosa | ANA specific for basal cells of stratified squamous epithelium |
| Linear IgA disease | Similar to erosive lichen planus | Linear deposits of IgA at the basement membrane zone | Same as for perilesional mucosa | IgA basement membrane zone (IgA) antibodies in 30% of cases |
| Dermatitis herpetiformis | Collection of neutrophils, eosinophils, and fibrin in the connective tissue papillae | IgA deposits in the dermal papillae in 85% of cases | IgA deposits in dermal papillae in 100% of cases | IgA endomysial antibodies in 70% of cases; gliadin antibodies in 30% of cases |
| Systemic lupus erythematosus | Hyperkeratosis, basal cell degeneration, epithelial atrophy, and perivascular inflammation | IgG or IgM with or without C3 deposits at the dermal–epidermal junction | Same as for perilesional mucosa | ANA in >95% of cases; DNA and ENA antibodies in >50% of cases |
| Chronic cutaneous erythematosus | Hyperkeratosis, basal cell degeneration, epithelial atrophy, and perivascular inflammation | IgG or IgM with or without C3 deposits at the dermal–epidermal junction | Negative | Usually negative |
| Subacute lupus erythematosus | Less inflammatory cell infiltrate than systemic and chronic cutaneous forms but with similar microscopic features | IgG or IgM with or without C3 deposits at the dermal–epidermal junction in 60% of cases; granular IgG deposits in basal cell cytoplasm in 30% of cases | Same as for perilesional mucosa | ANA in 60% to 90% of cases; Ro (SSA) in 80% of cases; RF in 30% of cases; anti-RNP in 10% of cases |
Modified from Rinaggio J, Neiders ME, Aguirre A, et al: Compendium 20:943, 1999.
Immunofluorescence.
For direct immunofluorescence, unfixed frozen sections are incubated with a variety of fluorescein-labeled, antihuman serum (i.e., anti-IgG, anti-IgA, anti-IgM, antifibrin, and anti-C3). With indirect immunofluorescence, unfixed frozen sections of oral or esophageal mucosa from an animal such as a monkey are first incubated with the patient’s serum to allow for the attachment of any serum antibodies to the mucosal tissue. The tissue is then incubated with fluorescein-labeled antihuman serum. Immunofluorescence tests are positive if a fluorescent signal is observed in the epithelium, its associated basement membrane, or the underlying connective tissue (see Table 19-1).
Management
In the first scenario, the dental practitioner takes direct and exclusive responsibility for the treatment of the patient; this occurs with conditions such as erosive lichen planus, which is responsive to topical steroids (Figure 19-3).
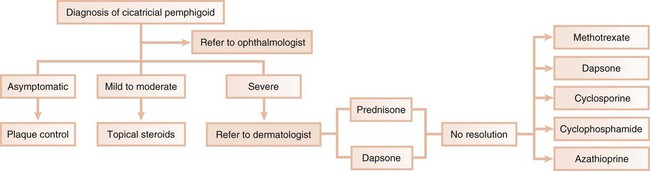
In the second scenario, the dentist collaborates with another health care provider to evaluate and treat a patient concurrently. The classic example is seen with cicatricial pemphigoid, in which dentists and ophthalmologists work together (Figure 19-4). Although the dentist addresses the oral lesions, the ophthalmologist monitors the integrity of the ocular conjunctiva.
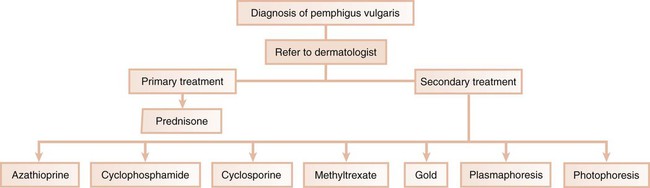
In the third scenario, the patient is immediately referred to a dermatologist for further evaluation and treatment. This occurs with conditions in which the systemic impact of the disease transcends the boundaries of the oral cavity and results in significant morbidity and even mortality. Pemphigus vulgaris is a clear example of a condition that, after diagnosis by the dentist, requires immediate referral to a dermatologist (Figure 19-5). In addition, the complications of chronically administered systemic medications that are indicated for the management of diseases such as pemphigus vulgaris or nonresponsive mucous membrane pemphigoid (e.g., diabetes mellitus, osteoporosis, methemoglobinemia) warrant referral to a dermatologist or a specialist in internal medicine.
Table 19-2 summarizes suggested contemporary therapeutic approaches that can be used to treat select conditions that clinically present as desquamative gingivitis. Dentists clearly play an important role in the diagnosis and management of desquamative gingivitis. The importance of being able to recognize and properly diagnose this condition is accentuated by the fact that a serious and life-threatening disease (e.g., squamous cell carcinoma) may mimic desquamative gingivitis.127
TABLE 19-2
Accepted Contemporary Therapeutic Approaches Used to Treat Select Conditions that Present Clinically as Desquamative Gingivitis
| Disease | Therapy |
| Erosive lichen planus | Mild cases: Delivery of therapeutic agent can be enhanced with use of vacuum-formed custom trays |
| Rx: Lidex (0.05% fluocinonide) gel | |
| Disp: One tube (1.5 g) | |
| Sig: Apply to affected area pc and hs | |
| Monitoring of the patient’s oral cavity is warranted because candidiasis may develop after a few weeks of topical steroid use; concomitant use of antifungal may be necessary | |
| Rx: Nystatin oral pastilles (100,000 IU) | |
| Disp: 60 pastilles | |
| Sig: Dissolve in mouth bid, then expectorate for 30 consecutive days | |
| Recalcitrant cases: | |
| Rx: Protopic (0.1% tacrolimus) ointment | |
| Disp: One tube (15 g) | |
| Sig: Apply to affected area bid | |
| Severe or refractory cases: Refer to dermatologist for management with systemic corticosteroids | |
| Cicatricial pemphigoid | Mild cases: |
| Rx: Lidex (0.05% fluocinonide) gel | |
| Disp: One tube (1.5 g) | |
| Sig: Apply to affected area pc and hs | |
| Rx: Temovate (0.05% clobetasol propionate) | |
| Disp: One tube (1.5 g) | |
| Sig: Apply to affected area qid | |
| Severe or refractory cases: Refer to dermatologist for management with prednisone (20 to 30 mg/day); concomitant use of azathioprine may be needed; dapsone, sulfonamide, and tetracycline are other alternatives | |
| Pemphigus | Refer to dermatologist for management with prednisone (20 to 30 mg/day); concomitant use of azathioprine may be needed |
| Chronic ulcerative stomatitis | Mild cases: |
| Rx: Lidex (0.05% fluocinonide) gel | |
| Disp: One tube (1.5 g) | |
| Sig: Apply to affected area qid | |
| Rx: Temovate (0.05% clobetasol propionate) | |
| Disp: One tube (1.5 g) | |
| Sig: Apply to affected area qid |
Diseases that Present Clinically as Desquamative Gingivitis
Lichen Planus
Lichen planus is an inflammatory mucocutaneous disorder that may involve the mucosal surfaces (e.g., oral cavity, genital tract, other mucosae) and the skin (including the scalp and the nails).131 Current evidence suggests that lichen planus is an immunologically mediated mucocutaneous disorder in which host T lymphocytes play a central role.11,64,92 Although the oral cavity may present lichen planus lesions with a distinct clinical configuration and distribution, the clinical presentation may sometimes simulate other mucocutaneous disorders. Therefore, a clinical diagnosis of oral lichen planus should be accompanied by a broad differential diagnosis. Numerous epidemiologic studies have shown that oral lichen planus presents in 0.1% to 4% of the population.139,142 The majority of patients with oral lichen planus are middle-aged and older women, with a 2 : 1 ratio of women to men. Children are rarely affected. In a dental setting, cutaneous lichen planus is observed in about one third of patients diagnosed with oral lichen planus.88 By contrast, two thirds of patients seen in dermatologic clinics exhibit oral lichen planus.136
Oral Lesions.
The erosive subtype of lichen planus is often associated with pain. It clinically manifests as atrophic, erythematous, and often ulcerated areas. Fine white radiating striations are observed bordering the atrophic and ulcerated zones. These areas may be sensitive to heat, acid, and spicy foods (Figure 19-6).
Gingival Lesions.
Approximately 7% to 10% of patients with oral lichen planus have lesions restricted to the gingival tissue.99,136 These may occur as one or more types of the following four distinctive patterns:
1. Keratotic lesions. These raised white lesions may present as groups of individual papules, linear or reticular lesions, or plaquelike configurations.
2. Erosive or ulcerative lesions. These extensive erythematous areas with a patchy distribution may present as focal or diffuse hemorrhagic areas. These lesions are exacerbated by slight trauma (e.g., toothbrushing) (Figure 19-7).
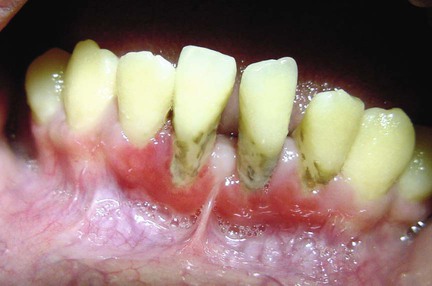
3. Vesicular or bullous lesions. These raised, fluid-filled lesions are uncommon and short lived on the gingiva, quickly rupturing and leaving ulcerations.
4. Atrophic lesions. Atrophy of the gingival tissues with ensuing epithelial thinning results in erythema that is confined to the gingiva.
Immunopathology.
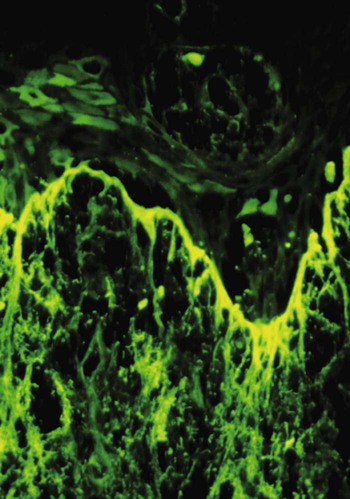
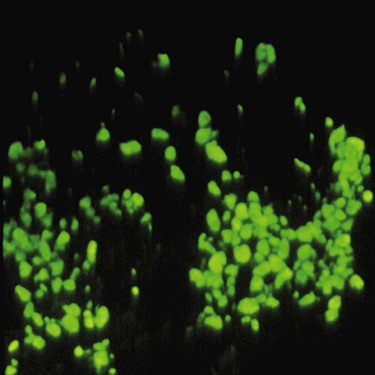
Direct immunofluorescence of both lesional and perilesional oral lichen planus biopsy specimens reveals linear fibrillar (“shaggy”) deposits of fibrin in the basement membrane zone (Figure 19-9), along with scattered immunoglobulin-staining cytoid bodies in the upper areas of the lamina propria (Figure 19-10). Serum tests involving the use of indirect immunofluorescence are negative in patients with lichen planus (see Table 19-1).
Treatment.
By contrast, the erosive, bullous, or ulcerative lesions of oral lichen planus are treated with high-potency topical steroids, such as 0.05% fluocinonide gel (Lidex, three times daily). Lidex can also be mixed 1 : 1 with carboxymethylcellulose (Orabase) paste or another adhesive ointment. A gingival tray can also be used to deliver Lidex or 0.05% clobetasol propionate with 100,000 IU/ml of nystatin in Orabase. Three 5-minute applications of this mixture daily appear to be effective for controlling erosive lichen planus.57 Intralesional injections of triamcinolone acetonide (10 mg to 20 mg) or short-term regimens of 40 mg of prednisone daily for 5 days, followed by 10 mg to 20 mg daily for an additional 2 weeks, have also been used in more severe cases.112 Because of the potential side effects, the administration of systemic steroids should be prescribed and monitored by a dermatologist. Other treatment modalities (e.g., retinoids, hydroxychloroquine, cyclosporine, azathioprine, cyclophosphamide, free gingival grafts) have also been used.112,119 A promising therapeutic agent, tacrolimus (0.1% Protopic ointment, twice daily), is an immunosuppressant that is effective for controlling the lesions of erosive lichen planus.72,91,104,144
Because candidiasis is often associated with symptomatic oral lichen planus and because topical steroid therapy promotes fungal growth, treatment should also include a topical antifungal agent.16,52,67
Pemphigoid
The term pemphigoid applies to a number of cutaneous, immune-mediated, subepithelial bullous diseases that are characterized by a separation of the basement membrane zone, including bullous pemphigoid, mucous membrane pemphigoid, and pemphigoid (herpes) gestationis.118,140 Among these conditions, bullous pemphigoid and mucous membrane pemphigoid (also known as benign mucous membrane pemphigoid and cicatricial pemphigoid), have received considerable attention. Current molecular findings involving these two diseases clearly indicate that they are separate entities.140 However, considerable histologic and immunopathologic overlap exists between the two diseases, so their differentiation may be impossible on the basis of these two criteria.118 In many cases, the clinical findings are probably the best cognitive element to use to discriminate between them. Accordingly, the term bullous pemphigoid is preferred when the disease is nonscarring and mainly affects the skin. The term cicatricial pemphigoid is favored when scarring occurs and the disease is mainly confined to mucous membranes, although scarring may be absent with some subtypes of mucous membrane pemphigoid.158 Until more research allows for the better understanding of this family of diseases, bullous pemphigoid and mucous membrane pemphigoid are discussed separately.
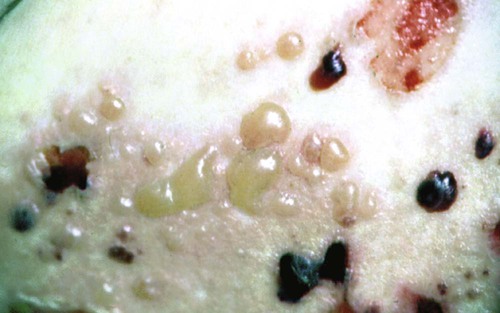
Bullous Pemphigoid.
Bullous pemphigoid is a chronic, autoimmune, subepidermal bullous disease with tense cutaneous bullae that rupture and become flaccid (Figure 19-11). Oral involvement occurs in about a third of affected patients.148
Immunofluorescence.
Immunologically, bullous pemphigoid is characterized by immunoglobulin G (IgG) and complement 3 (C3) immune deposits along the epithelial basement membranes and circulating IgG antibodies to the epithelial basement membrane.71,109 Direct immunofluorescence is positive in 90% to 100% of these patients, whereas indirect immunofluorescence is positive in 40% to 70% of affected patients110 (see Table 19-1).
Treatment.
Because its etiologic factors are unknown, the treatment of bullous pemphigoid is designed to control its signs and symptoms.71,109 The primary treatment is a moderate dose of systemic prednisone. Steroid-sparing strategies (i.e., prednisone plus other immunomodulatory drugs) are used when high doses of steroids are needed or when the steroid alone fails to control the disease.38 For localized lesions of bullous pemphigoid, high-potency topical steroids or tetracycline with or without nicotinamide can be effective.112
Mucous Membrane Pemphigoid (Cicatricial Pemphigoid).
Mucous membrane pemphigoid, which is also known as cicatricial pemphigoid, is a chronic vesiculobullous autoimmune disorder of unknown cause that predominantly affects women during the fifth decade of life. It has also rarely been reported in young children.22,106,140
Cicatricial pemphigoid involves the oral cavity, the conjunctiva, and the mucosa of the nose, vagina, rectum, esophagus, and urethra. In about 20% of patients, however, the skin may also be involved. Recent investigations suggest that cicatricial pemphigoid encompasses a group of heterogeneous conditions with distinct clinical and molecular features.33,101,133 An elaborate cascade of events is involved in the pathogenesis of cicatricial pemphigoid. Initially, antigen–antibody complexing occurs at the basement membrane zone, and this is followed by complement activation and subsequent leukocyte recruitment. Next, proteolytic enzymes are released and dissolve or cleave the basement membrane zone, usually at the level of the lamina lucida.45 The two major antigenic determinants for cicatricial pemphigoid are bullous pemphigoid 1 and 2 (BP1 and BP2). Most cases of cicatricial pemphigoid are the result of an immune response directed against BP2; less often, this response is mounted against BP1, epiligrin (laminin-5, a lamina lucida protein in basement membrane of stratified epithelium), and β4 integrins.8,20,33
Current research strongly suggests that there are at least five subtypes of cicatricial pemphigoid: oral pemphigoid, anti-epiligrin pemphigoid, anti-BP antigen mucosal pemphigoid, ocular pemphigoid, and multiple-antigens pemphigoid.140 Recent studies have shown that the sera of ocular pemphigoid patients recognize the β4 integrin subunit, whereas the sera of patients with oral pemphigoid recognize the βα6 unit.124
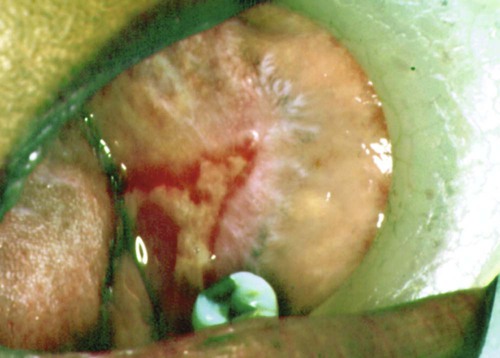
Ocular Lesions.
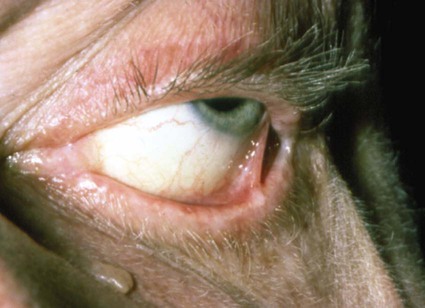
Among patients who first present to the dentist (mainly with desquamative gingivitis), the eyes are affected in approximately 25%.110 By contrast, among patients who first present to the dermatologist, 66% present with conjunctival lesions; in ophthalmic studies, 100% of patients have ocular involvement.51,81,102,103 The initial lesion is characterized by unilateral conjunctivitis that becomes bilateral within 2 years. Subsequently, there may be adhesions of eyelid to eyeball (symblepharon) (Figure 19-12). Adhesions at the edges of the eyelids (ankyloblepharon) may lead to a narrowing of the palpebral fissure. Small vesicular lesions may develop on the conjunctiva, which may eventually produce scarring, corneal damage, and blindness.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses