Chapter 15
Diagnosis and management of endodontic complications after trauma
Introduction
Blows to the mouth can transmit sufficient energy to fracture teeth, displace teeth or fracture alveolar bone. The immediate consequences may appear obvious and dramatic (Fig. 15.1) or seemingly quite trivial, but in many circumstances, the true picture takes time to emerge. Complications can be serious in the long term, resulting not only in the loss of vital pulp functions, but in the gradual or rapid destruction of tooth structure by resorptive processes. Clinicians should consequently be aware that their emergency care must be followed by a structured program of review appointments, if complications are to be identified early and treated with success. The purpose of this chapter is to outline important considerations in the management of teeth which have suffered trauma. It will examine how trauma can make the pulp vulnerable to breakdown and infection, how trauma complicates pulp diagnosis and treatment planning and how pulp infection may combine with other injuries to promote secondary conditions such as root resorption. Some of the endodontic and restorative challenges presented by traumatized teeth with immature, fractured or resorbed roots will also be discussed.
Fig. 15.1 The consequences of trauma may appear obvious, but time may reveal lesser injuries, including those in adjacent teeth, to be equally serious in the long term. (Courtesy of Dr Ben Cole.)
Common dental injuries
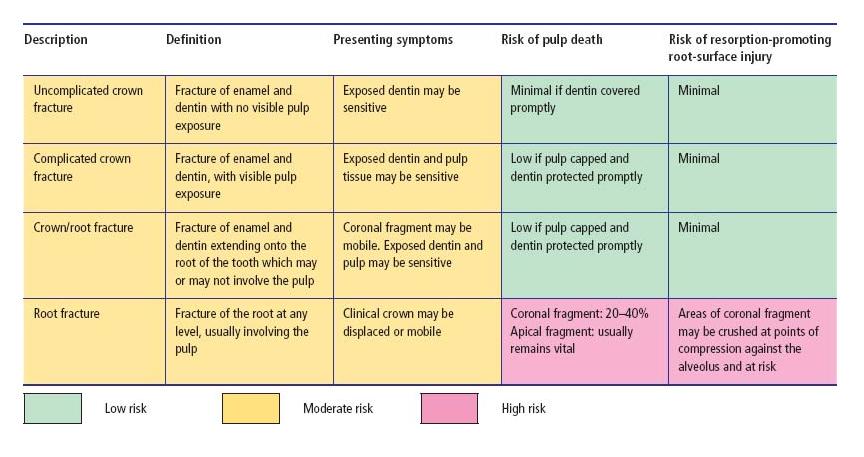
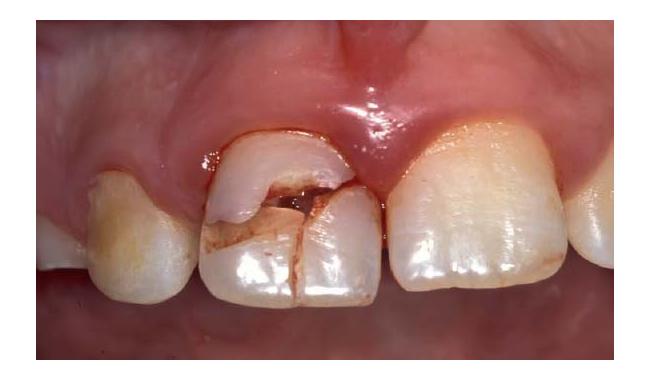
Traumatic injuries to the teeth include fractures, luxations and combinations of the two. Tooth fractures may be confined to the structure of the crown or the root, or involve both. They may be classified according to their position, the degree of tooth tissue loss and whether or not they are complicated by direct exposure of pulp tissue to the oral environment (see Table 15.1 and Fig. 15.2). Intra-alveolar “horizontal” root fractures often run obliquely, but are usually classified according to their horizontal level as apical, mid-root or coronal third fractures (Fig. 15.2d). Horizontal root fractures detach the coronal part of the tooth from the remainder of the root, and may potentially disrupt the pulp’s neurovascular supply at the fracture line, though they rarely expose pulp tissue to the mouth. Fractures may be undisplaced and difficult to identify with certainty (Fig. 15.2e), but if the crown is also luxated, the fracture will be displaced and more readily identified (Fig. 15.2f).
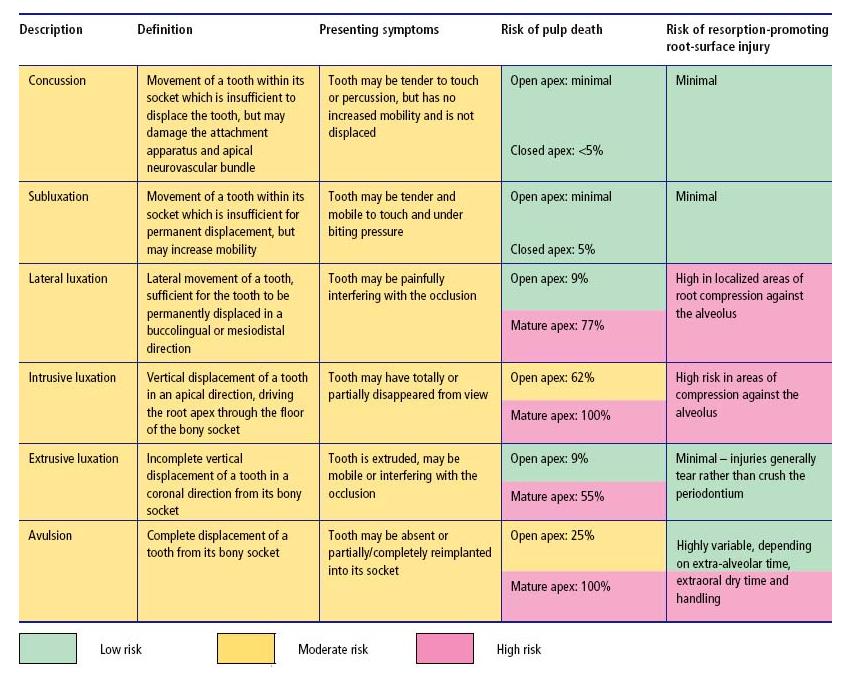
Luxation is an injury where the tooth has been loosened from its alveolus to a varying extent (see Table 15.2 and Fig. 15.3). Following minor injury, there may be no permanent dislocation of the tooth. More severe injuries can dislocate teeth in a lateral, apical or coronal direction or even displace them completely from the alveolus (avulsion) (Fig. 15.3d).
Table 15.1 Tooth fractures – classification, clinical features and risks of complication (see also Fig. 15.2). Percentages derived from Andreasen et al. (7).
Fig. 15.2 Fractured teeth. (a) Uncomplicated crown fracture. (b) Complicated crown fracture. (c) Crown–root fractures, which may or may not directly involve the pulp. (d) Intra-alveolar “horizontal” root fractures in the coronal, middle or apical root third. Although the pulp is invariably involved in the fracture line, it may not be directly exposed to the oral environment. (e) Undisplaced intra-alveolar “horizontal” root fracture. Radiographically (inset) the fracture may be difficult to detect, or may be misinterpreted as more than one fracture line with an apparent island of tooth tissue interposed between them. (f) Displaced intra-alveolar fracture following concomitant luxation and displacement of the crown.
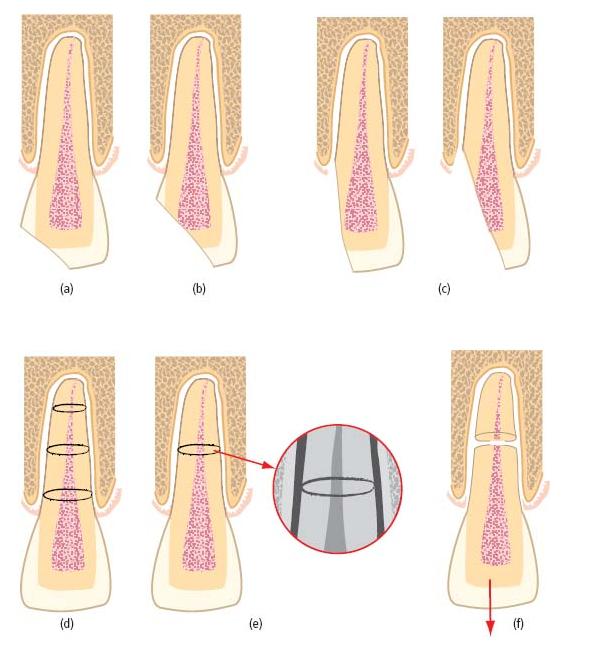
Table 15.2 Tooth luxation – classification, clinical features and risks of complication (see also Fig. 15.3). Percentages derived from Andreasen et al. (7).
Fig. 15.3 Luxated teeth. (a) Lateral luxation – may sever the pulp’s neurovascular supply and compress areas of the root against the alveolus. (b) Intrusive luxation – invariably disrupts the pulp’s neurovascular supply and drives the root end into the alveolus. (c) Extrusive luxation – may disrupt the pulp’s neurovascular supply; periodontal fibers are torn rather than crushed. (d) Avulsion – complete loss of the tooth from the alveolus severs the pulp’s neurovascular supply and exposes the root surface to considerable risk of damage.
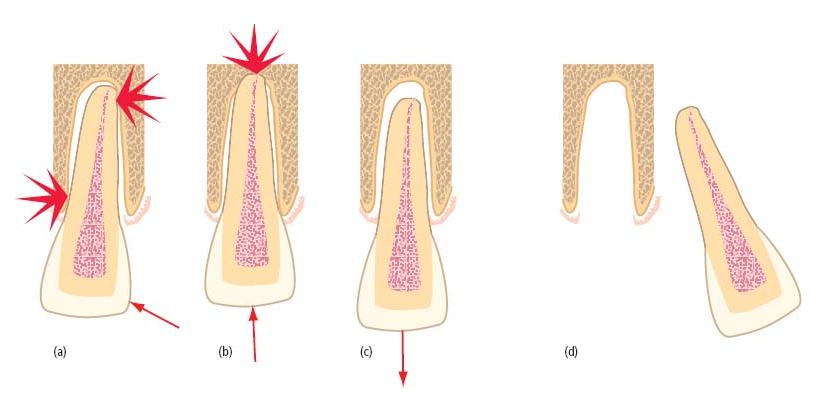
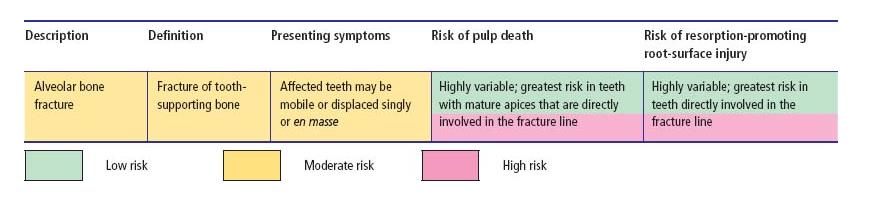
Table 15.3 Alveolar fracture – classification, clinical features and risks of dental complication.
Fractures to the alveolar process may cause individual or groups of teeth to become mobile, disrupt the neurovascular supply to their pulps resulting in transient or permanent loss of pulp functions (Table 15.3), or damage the surface of their roots.
The following sections will examine in greater detail how different types of injury endanger the pulp and integrity of the root surface, and how unwanted complications may arise.
Dental trauma and its consequences
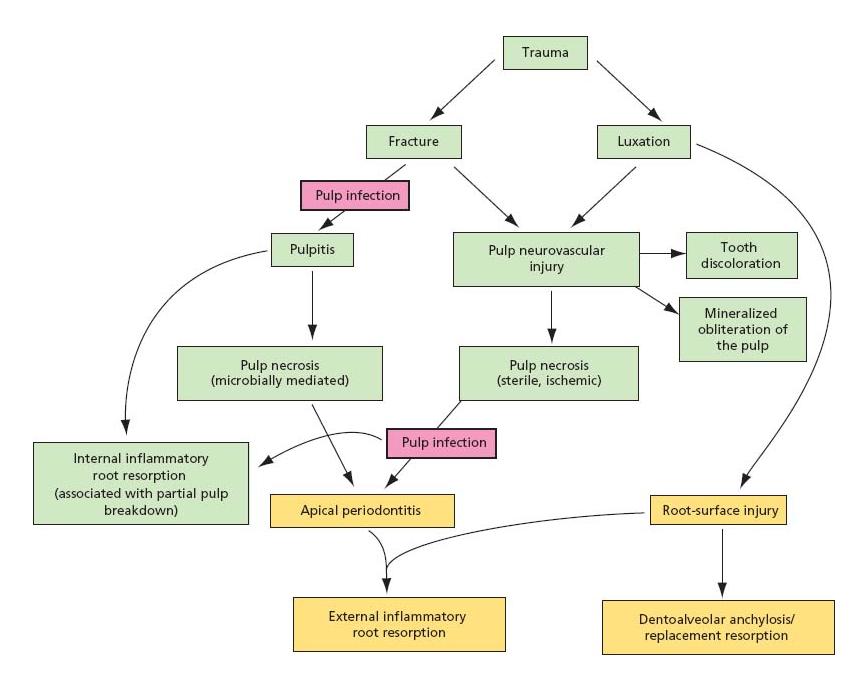
Flow diagram 15.1 provides a schematic overview of the complications, which may follow traumatic dental injuries.
Flow diagram 15.1 Schematic representation of complications after traumatic dental injury.
Traumatic injuries and the pulp
Both fracture and luxation injuries can have serious implications for the short- and long-term functions of the pulp (see Core concept 15.1).
Fractures
Uncomplicated crown fractures (Table 15.1, Fig. 15.2a) expose dentinal tubules to the mouth and create a potential gateway for pulp infection. The pulp is often in a healthy condition at the time of injury, and defensive mechanisms within the dentin–pulp complex protect the pulp from significant microbial invasion provided the exposed tissue is appropriately covered. More severe fractures which directly expose the pulp to the oral environment (Fig. 15.2b, c) may risk significant pulp infection and breakdown, but if properly managed by conservative non-invasive treatment (see further below and Chapter 4), there is a good chance of long-term pulp survival.
Luxations
On the other hand, luxation injuries frequently threaten the vital functions of the pulp. Neurovascular bundles are often torn when teeth are displaced or involved in fractures. At its most extreme, this may cause immediate, avascular necrosis of pulp tissue lying peripheral to the neurovascular tear (Fig. 15.4). Less severe disruption may cause only temporary ischemic injury of the pulp, but this may nevertheless reduce its defensive capacity and leave it vulnerable to otherwise trivial microbial challenges. Finally, an altered neurological response may complicate pulp diagnosis and result in the unnecessary extirpation of a healthy but non-responsive pulp (see further below, Diagnostic quandaries).
Another cause of neurovascular damage is from internal bleeding, due to rupture of the larger blood vessels in the pulp tissue per se. The bleeding reaction, if extensive, may result not only in reddening and later graying of the crown (38), but in breakdown of the entire tissue within a very short period of time.
Fig. 15.4 Avascular necrosis after serious disruption of the pulp’s neurovascular supply. (a) Complete, after displacement of a tooth with a mature apex. In teeth with open, immature apices, there is a reasonable prospect that the pulp may revascularize and recover. (b) Partial, after horizontal root fracture. Coronal pulp breakdown is not inevitable, but even if the pulp tissue in the coronal portion loses vitality, the apical fragment usually remains vital.
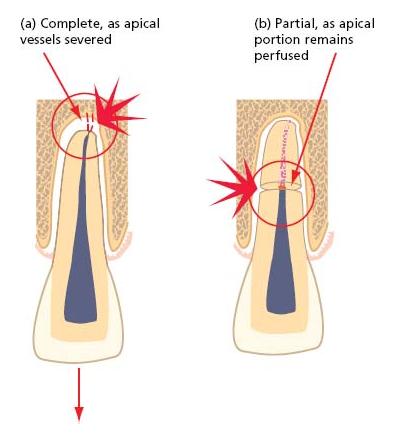
Only in teeth with immature, open apices can pulp revascularization be expected after significant displacement or avulsion (Table 15.2). Teeth with mature apices have limited capacity for pulp regeneration through the narrow apical foramen and complete, avascular pulp necrosis is almost certain after avulsion or intrusive luxation, and highly likely after extrusive or lateral luxation.
The prospects of pulp revascularization are generally higher in both mature and immature teeth after horizontal root fracture, and the well-perfused pulp of the apical fragment invariably remains vital even if complete pulp breakdown occurs in the coronal fragment. The level of the fracture line may have a significant bearing on outcome, with the greatest risk of coronal pulp breakdown after fractures involving the coronal third of the root (see Key literature 15.1).
Sterile, necrotic pulps may persist quietly in that state for many years, and are incapable of initiating or propagating apical periodontitis (9, 44). They do, however, provide a nutritious environment for microbial colonization, an event which will trigger the development of periapical inflammation and its consequences. In an apparently intact tooth with no obvious fractures, the likelihood of microorganisms invading the necrotic pulp may seem remote. But without a constant outflow of dentinal fluid, dentinal tubules present defenseless portals for microbial entry through exposed dentin surfaces, around restoration margins and even through microcracks in the overlying enamel (27). Direct pulp exposure allows unimpeded access for the oral flora. More contentious is the concept of anachoresis, where microorganisms gaining entry to the circulation from a variety of body sites (including the oral cavity and the gut) may be transported and seeded into areas of necrotic pulp tissue (37). It is rare for organisms other than oral residents to be recovered from infected pulp canals, suggesting that this may not be a major route of colonization (see Chapter 6).
Obliteration of the pulp space by mineralized tissue
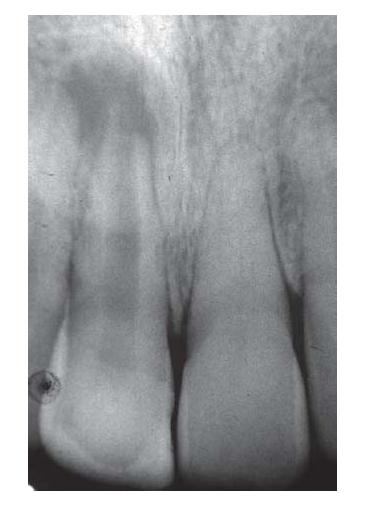
Pulp volume diminishes throughout life by the physiological apposition of secondary dentin and by the deposition of tertiary dentin in response to localized insults. An additional and distinctive mineralized response is the accelerated formation of hard tissue after trauma; this may partially or completely obliterate the pulp space, particularly of young teeth (1). The incidence is highest after luxation injuries and changes may be identified as early as 3 months after injury, though 12 months is more common (Fig. 15.5; see also Fig. 2.9) (1). The mechanisms of this response are incompletely understood, though it has been postulated that odontoblasts, and perhaps other mesenchymal cell populations within the pulp, may lose autonomic regulatory control during neurovascular injury and regeneration, resulting in accelerated and disorganized hard-tissue deposition.
Histopathologically, the tissue often resembles osteodentin with an irregular tubular pattern and a maze of spaces and cul-de-sacs containing pulp cells which have become trapped within the rapidly deposited material (1). Clinically, the tooth may take on a yellow, or occasionally gray, hue as a result of the thickened and less translucent coronal dentin.
Fig. 15.5 Accelerated pulp obliteration by mineralized tissue in tooth 21 after trauma. The adjacent tooth 11 suffered pulp necrosis and arrested root development (see also Fig. 2.9).
From a practical standpoint, it is important to understand whether pulp canal obliteration represents a degenerative change, leading ultimately to pulp breakdown and infection, and whether interventions to improve the appearance of the tooth such as orthodontic movement, veneer or crown preparation make these events more likely. A perennial question, which flows from this, is whether prophylactic root canal treatment is indicated to prevent apical periodontitis in all teeth that show signs of pulp space obliteration. A related question is whether there is any realistic hope of successfully treating pulp infection in an obliterated pulp space. As a worst-case scenario, Jacobsen and Kerekes (24) (see Key literature 15.2) observed apical periodontitis on 21% of teeth with complete pulp obliteration during a 10–23-year review period. They concluded that routine prophylactic root canal treatment could not be justified in all teeth showing signs of pulp space obliteration. Similar conclusions were drawn by Robertson et al. in 1996 (36); they found evidence of pulp necrosis in only 8.5% of teeth with pulp canal obliteration during a mean observation period of 16 years. This study provided further insight into the impact of caries, new trauma, orthodontic treatment and crown/veneer preparation on pulp survival. None of these additional insults had a significant impact on pulp survival, and it was concluded that, although the incidence of pulp necrosis increased with time in teeth with pulp canal obliteration, routine prophylactic endodontics did not seem justified (1, 36).
In the event of pulp breakdown, clinical data from the early 1980s (16) suggest that root canal treatment is possible and can result in complete healing in 80% of cases. The situation may well have improved in the light of contemporary microscope-assisted endodontic practice for canal detection, and developments in endodontic microsurgery may have created additional possibilities for the successful management of teeth which cannot be treated non-surgically.
Consequences of pulp breakdown and infection after trauma
Key endodontic consequences of pulpal necrosis and subsequent infection after trauma include inflammatory bone resorption, inflammatory root resorption and the arrest of dental development.
Inflammatory bone resorption
As described in Chapter 7, infected pulps cause inflammatory lesions in the alveolar bone adjacent to any portal of pulp–periodontal communication. It is critical to note that teeth damaged by trauma may present new pathways of communication which did not exist previously, such as fractures through the body of the root and tubular communications through dentinal tubules which have been made patent by the loss of root-surface cementum. Damage to the root surface is least likely when the periodontal ligament is stretched or torn, and most likely when the periodontium is crushed, dehydrated, handled or heavily soiled. Tables 15.1–15.3 present the likely risks of root-surface injury associated with different categories of trauma. Tooth avulsions, which expose the whole root surface to injury (Fig. 15.3d), and lateral and intrusive luxations which drive areas of the root against the walls of the alveolar socket (Fig. 15.3a, b) present the greatest risk. Open dentinal tubules, combined with an infected pulp, provide the conditions for periradicular inflammation in the bone adjacent to the open tubules, and for external inflammatory root resorption, a particularly damaging and unwanted complication.
Inflammatory root resorption
While alveolar bone exists in a state of constant remodeling, the intact teeth which it supports are usually unaffected. The most likely explanation is that incompletely mineralized layers of predentin and precementum cover the internal and external surfaces of teeth, respectively, and inhibit resorptive activity (47). Injuries, which cause these surfaces to be damaged, lost or mineralized make them vulnerable to resorption, as mononuclear phagocytes rapidly migrate to the area and coalesce to form multinuclear clastic cells of the monocyte lineage, termed “odontoclasts”. Resorption is likely to commence soon after, but may be short-lived. The activity of clastic cells requires constant stimulation and in the absence of a propagating stimulus, the resorption will be only “transient” (46). These “surface” resorption defects arrest within 2–3 weeks and are repaired by cementum-like material. If, on the other hand, there is a propagating stimulus, such as periradicular inflammation caused by pulp–space infection (Fig. 15.6a), resorption is likely to progress: “external inflammatory root resorption”. Hard tissue can be destroyed rapidly and there is great danger in watching and waiting if areas of external root resorption are identified on teeth with non-vital pulps. Removal of the propagating stimulus predictably arrests external inflammatory root resorption. Cvek (14) reported 97% success after treatment, which included an interim calcium hydroxide dressing. The need for intracanal medication with calcium hydroxide is by no means certain, however (14).
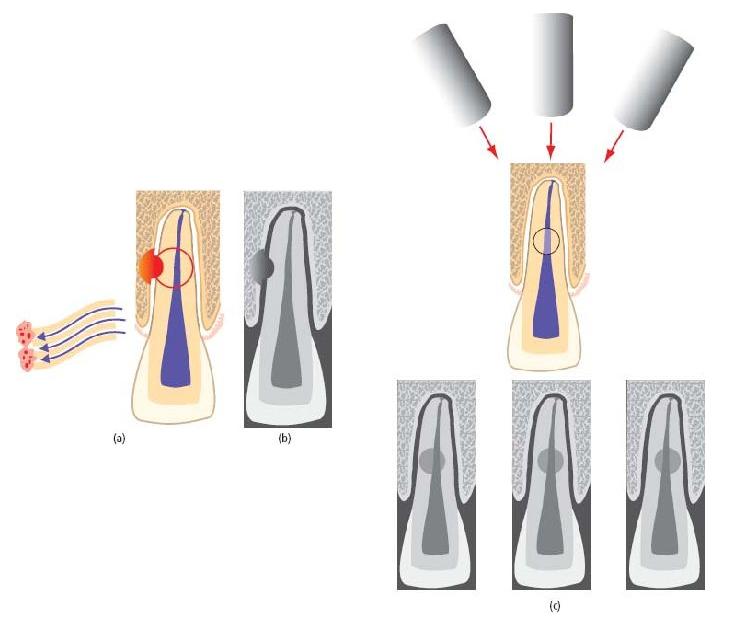
External inflammatory root resorption is usually identified on routine radiographic review as punched-out areas of hard-tissue loss from the root surface (Fig. 15.6b), associated with radiolucent areas in the adjacent alveolar bone. Lesions on the labial or lingual root surface can be confused with internal resorption (see Fig. 15.8), but a change in the radiographic angle usually reveals that the lesion moves in relation to the root canal, and the lateral margins of the pulp canal are usually superimposed (Fig. 15.6c). Exciting developments in the diagnostic imaging of resorptive lesions (13, 33) are introduced in Advanced concept 15.1.
Fig. 15.6 External inflammatory root resorption. (a) Propagated by an infected, necrotic pulp. Trauma has resulted in concomitant root surface injury and pulp breakdown. Secondary infection of the pulp provides the propagating stimulus necessary for progressive resorption, microbial elements diffusing to the root surface through patent dentinal tubules. (b) A characteristically punched-out area the of root surface with an associated bone radiolucency. (c) Labially or palatally placed lesions shift with changes of radiographic angle and may be superimposed by the “tram-lines” of the pulp canal walls.
External inflammatory root resorption can arise at any level of the root surface and may present in the cervical region where it communicates with the oral environment: “cervical” or “extracanal invasive” resorption (Fig. 15.7a). Although the pathogenesis is incompletely understood, traumatic injury to the cervical part of the root has been suggested as a risk factor (23), and the infected pulp space may again serve as the propagating stimulus. It must, however, be borne in mind that such lesions may also occur in teeth which have no known history of trauma and in teeth with healthy pulps. Some considerations in the clinical management of cervical resorption are outlined in Advanced concept 15.2.
Internal inflammatory root resorption
A similar process, internal inflammatory root resorption, may arise within a tooth after damage or mineralization of areas of predentin lining the pulp chamber (Fig. 15.8). This could arise after a blow which causes intrapulpal hemorrhage and localized compression with death of odontoblasts. Damaged surfaces, or surfaces which have become mineralized following direct exposure to tissue fluids, are again believed to be colonized by clastic cells derived from mononuclear phagocytes (50), which begin the process of transient (internal) root resorption. In many circumstances, the entire pulp will proceed to break down, denying nutrition to the clastic cells and arresting the resorptive process. In others, the pulp may recover, and in the absence of a propagating stimulus, resorption would be expected to cease within 2–3 weeks.
Fig. 15.7 Cervical resorption. (a) A characteristically placed lesion, which may be visible as a pink discoloration through the crown. The external entry point may be small, though the resorption burrows extensively within dentin. (b) Radiographic appearance. In this case, the lesion is extensive and has broad communication with the marginal periodontium, necessitating open debridement and restoration.
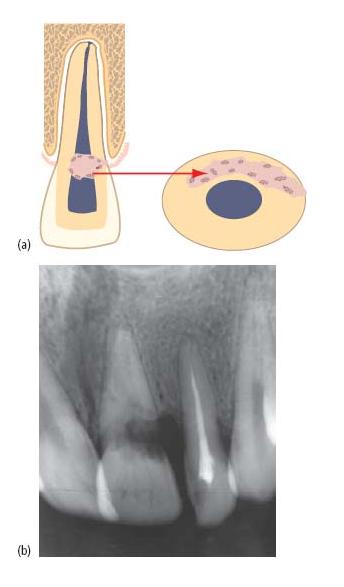
On rare occasions, the pulp may partially break down, with infected, necrotic pulp tissue coronally and vital pulp tissue persisting apically. Tissue at the interface persists in an inflamed state, under the influence of microbial elements within the necrotic coronal pulp. It has been postulated that branching, transverse communications between dentinal tubules may provide a pathway for microbial products to act as a propagating stimulus for progressive internal inflammatory root resorption (Fig. 15.8a) (46). This form of resorption expands the pulp canal in a characteristically symmetrical, ballooning fashion. Internal root resorptions may be detected as a chance radiographic finding, or present with painful symptoms when the lesion has perforated the root surface. Occasionally, lesions affecting the coronal third of the root may cause pink discoloration of the crown.
Internal and external inflammatory root resorptions can be confused radiographically, though it is unusual to observe the lateral margins of the pulp canal superimposed on an internal resorption. Additionally, since internal resorption represents expansion of the canal, its relationship is unlikely to change with alterations of radiographic angle (Fig. 15.8a).
Fig. 15.8 Internal inflammatory root resorption. (a) Characteristic, symmetrical expansion of the pulp space is noted at the interface between necrotic and vital tissue. The resorbed area has a constant relationship to the pulp space with alteration of the radiographic horizontal angle. There are no superim/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses