Nonsurgical Periodontal Therapy
Phyllis L. Beemsterboer and Dorothy A. Perry
• Define nonsurgical periodontal therapy.
• Describe the short- and long-term goals of nonsurgical periodontal therapy.
• Identify the techniques and applications for nonsurgical periodontal therapy procedures.
• Describe the process of healing after periodontal debridement procedures, scaling, and root planing.
• Explain the limitations of calculus removal and the expectations for clinician proficiency.
• Discuss the use of lasers in nonsurgical therapy.
• Describe the contributions of magnification with use of loupes, endoscopy, and microscopes to nonsurgical therapy.
• Explain the benefits and indications of antimicrobial adjuncts to nonsurgical therapy.
The initial approach for treating gingival and periodontal diseases is debridement of plaque biofilm and calculus through nonsurgical therapeutic techniques. The term nonsurgical therapy is often considered a misnomer because the procedures performed require the application of sharp blades to cut tissues, which is a form of surgery. However, in periodontology, the term surgery is reserved for more invasive cutting procedures. Other terms used to describe nonsurgical periodontal therapy include initial therapy,1 Phase I therapy,2,3 etiotropic phase,2 and periodontal debridement.
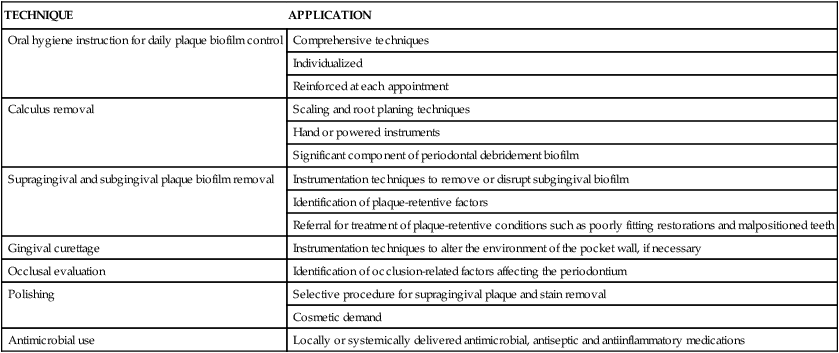
In its broadest sense, nonsurgical therapy defines all of the procedures performed to treat gingival and periodontal diseases up to the time of reevaluation, which is when patients begin maintenance care and the need for periodontal surgery to enhance results is determined. Nonsurgical therapy includes the procedures listed in Table 13-1.
TABLE 13-1
Nonsurgical Periodontal Therapy Appointment Procedures
| TECHNIQUE | APPLICATION |
| Oral hygiene instruction for daily plaque biofilm control | Comprehensive techniques |
| Individualized | |
| Reinforced at each appointment | |
| Calculus removal | Scaling and root planing techniques |
| Hand or powered instruments | |
| Significant component of periodontal debridement biofilm | |
| Supragingival and subgingival plaque biofilm removal | Instrumentation techniques to remove or disrupt subgingival biofilm |
| Identification of plaque-retentive factors | |
| Referral for treatment of plaque-retentive conditions such as poorly fitting restorations and malpositioned teeth | |
| Gingival curettage | Instrumentation techniques to alter the environment of the pocket wall, if necessary |
| Occlusal evaluation | Identification of occlusion-related factors affecting the periodontium |
| Polishing | Selective procedure for supragingival plaque and stain removal |
| Cosmetic demand | |
| Antimicrobial use | Locally or systemically delivered antimicrobial, antiseptic and antiinflammatory medications |
This chapter defines the technical procedures applied by dental hygienists and the instruments used for treatment. These are the procedures and instruments required to scale, root-plane, and debride the tooth surfaces of bacterial plaque biofilms and calculus and to remove stains by the application of polishing techniques.3
Patient plaque biofilm control is a cornerstone of long-term successful therapy. For this reason, every patient must participate in treatment by adopting a regular and effective biofilm removal regimen. Positive, long-term effects of periodontal therapy are reliably achieved with patient compliance, effective plaque biofilm control, and excellent dental hygiene treatment.3 These are all aspects of dental hygiene care and are essential in the application of nonsurgical periodontal therapy.
Definitions of Nonsurgical Periodontal Therapy
This chapter discusses the biologic basis and rationale for nonsurgical therapeutic procedures performed in the dental office. It describes scaling procedures, both hand instrumentation and powered instrumentation, root planing, gingival curettage, and polishing. Treatment frequently requires the use of pain control measures. Elements of dental hygiene care are illustrated in Figures 13-1 to 13-3.
Scaling
The American Academy of Periodontology (AAP) defines scaling as “instrumentation of the crown and root surfaces of the teeth to remove plaque, calculus, and stains from these surfaces.”4 However, subgingival scaling is also referred to as simply the removal of subgingival calculus3 or the more general term, subgingival deposits.5 Scaling is most commonly thought of as the removal of identifiable deposits of calculus, but associated plaque biofilm deposits are also removed during the procedure. The focus of the application of the instruments is to remove calculus and biofilms; success of treatment at the time of the therapy is assessed by explorer evaluation of root smoothness after scaling procedures to ensure calculus removal.
Root Planing
Root planing is defined by the AAP as “a treatment procedure designed to remove cementum or surface dentin that is rough, impregnated with calculus, or contaminated with toxins or microorganisms.”6 This procedure focuses not on identifiable deposits of calculus but on the entire root surface associated with the periodontal pocket. The goal of root planing is to remove the surface layer of cementum or dentin that may be impregnated with bacterial lipopolysaccharides (endotoxins) or calculus to create a glassy, hard surface.5 When the root surfaces feel smooth and hard, the dental hygienist can be confident that the treated pockets are free of deposits and contaminants on and embedded in the root surfaces.7 Root planing was thought to render root surfaces less prone to the reestablishment of the cause of disease—bacterial plaque biofilm—than scaling alone, but this theory has not been proven. The difference between scaling and root planing is a matter of degree; root planing involves a specific effort to instrument every portion of the root surfaces, not simply identifiable calculus deposits.
The goal of root planing, leaving the roots clean, has not changed, but the extent to which root tissue is scraped away to create a glassy, hard texture has been under scrutiny. There is no evidence that root-planed teeth are easier to maintain or less likely to be associated with periodontal diseases than those that have simply been rendered free of calculus and plaque biofilm.8
Periodontal Debridement
The terms nonsurgical periodontal therapy or periodontal debridement are used along with the traditional terms of scaling and root planing. These terms include supragingival and subgingival scaling and root planing and disruption or removal of plaque biofilm, with a minimum of tooth structure removal.4 They also incorporate removal of plaque biofilm, plaque retentive features, and calculus, both above and below the gingival margin. The goal of periodontal debridement and scaling is to restore the periodontium to health and produce surfaces that are free of various deposits.6,9
Prophylaxis
Prophylaxis is a preventive procedure to remove local gingival irritants and includes complete calculus removal followed by root planing. The term is commonly used and has several variations: oral prophylaxis, dental prophylaxis or, simply, prophy. The purpose of prophylaxis is to assist the patient in maintaining and preserving periodontal health. It is usually accomplished during one appointment and has many facets. A comprehensive explanation of periodontal maintenance is found in Chapter 17.
Polishing and Stain Removal
Stains on the teeth are generally considered harmless, so their removal is secondary to the therapeutic and preventive goals of the dental hygienist. Polishing should be performed selectively.10,11
Selective polishing is choosing the surfaces to polish on the basis of patient concerns and the presence of plaque biofilm and stains that cannot be removed with normal patient oral hygiene practices. The concept of selective polishing emerged when research on enamel and root surfaces after polishing revealed changes in the hard tissues. In 1976 Wilkins, in her fourth edition of Clinical Practice of the Dental Hygienist, introduced the idea of selective polishing and encouraged this modification in treatment.9 She stressed the critical importance of teaching personal plaque biofilm control rather than performing polishing during the appointment because of the limited amount of time the dental hygienist has with each patient. Providing information about performing effective plaque biofilm control is more valuable than performing what is primarily a cosmetic procedure.
Several other concerns about polishing exist. Abrasives used during polishing can scratch amalgam, composite resin, and gold restorative materials. It was once thought that tooth surfaces had to be plaque-free to absorb fluoride during fluoride treatments, so polishing of teeth was performed routinely before office fluoride applications. It is now known that the presence of plaque biofilms does not interfere with the uptake of fluoride by tooth structures. Other concerns include the possibility of creating bacteremia in the patient and possibly damaging the tooth pulps by heat generated from the power-driven prophylaxis angle. Cleaning agents are available for polishing the teeth and are preferable to those that contain abrasives. The contents of any material used for patient care should be read carefully; this is especially warranted when dealing with the myriad choices available for stain removal. Barnes recommended that the least abrasive paste necessary to remove stains was appropriate and if no stain was present a cleaning agent should be employed. One size fits all grit paste ignores the science of abrasion, can cause sensitivity, and damage aesthetic restorations.5
Air powder polishing removes most extrinsic stains and soft deposits from the exposed surfaces of the teeth. It works by mechanical abrasion using a slurry of sodium bicarbonate and water. The power and powder-to-water ratio is controlled with a foot pedal and can be increased or decreased as needed. Air powder polishing is especially effective with severe staining, such as that found in cigarette and pipe smokers. Caution must be exercised with this device to prevent damage to exposed root surfaces; thus, its application for periodontal patients is limited. Because this system produces an extensive aerosol, it is contraindicated in patients with infectious diseases, respiratory illnesses, hypertension, or those who are on hemodialysis.10 The periodontal patient often has multiple exposed root surfaces and caution with the choice of polishing agent is advised.
Gingival Curettage
Curettage had been defined by the AAP as scraping or cleaning the walls of a cavity or surface by means of a curette.12 It is a commonly misused term, often applied to a variety of procedures from removal of the pocket lining, termed closed curettage, to a surgical flap procedure called open curettage. Specifically, curettage performed by the dental hygienist (legally permitted in some states), properly termed gingival curettage, is limited to closed curettage. It is defined as the removal of the inflamed soft tissue lateral to the pocket wall. A number of clinical trials have confirmed that gingival curettage provides no additional benefit to healing compared with scaling and root planing alone in terms of probing depth reduction, attachment gain, or inflammation reduction. Gingival curettage is thus considered to have little therapeutic value in the treatment of chronic periodontitis and is no longer listed as a method of treatment by the American Dental Association and the AAP.12
Goals of Nonsurgical Periodontal Therapy
The goals of nonsurgical periodontal therapy must be considered in terms of the immediate treatment goals at the time of the appointment and the long-term goals for the patient. During periodontal debridement procedures, the goal for the dental hygienist is to promote plaque biofilm control and instrument the tooth surfaces until they are clean and smooth, touching all portions of the roots to disrupt plaque biofilm and remove calculus. This end point is best evaluated by explorer detection of smooth surfaces.3 Calculus removal may be considered a subgoal rather than the primary focus.3 The goal at the treatment visit is not to render the roots glassy and hard through extensive planing away of tooth structure.
The long-term goal of treatment is to restore gingival health. For periodontal patients, this goal often requires multiple appointments with the dental hygienist. The restoration of gingival health is the sum of good plaque control, complete scaling and periodontal debridement, and sufficient time for healing to occur—several months for complete healing of both the epithelium and connective tissue.2,3 These goals are summarized in Table 13-2.
TABLE 13-2
Goals of Nonsurgical Periodontal Therapy
| PLAQUE BIOFILM CONTROL | CALCULUS AND BIOFILM REMOVAL (PERIODONTAL DEBRIDEMENT) | EVALUATION | |
| Short-term goals | Provide technique instruction and reinforcement | Complete removal of deposits Clean surfaces |
Clean and smooth root surfaces |
| Long-term goals | Ensure adoption of adequate daily oral hygiene procedures Reinforcement |
Regular removal of new deposits at subsequent visits | Tissue health restored Compliance with maintenance regimen (regular evaluation and treatment as necessary) |

Rationale for Nonsurgical Periodontal Therapy
The rationale for nonsurgical periodontal therapy is to remove the etiologic agent of disease—bacterial plaque biofilm—and its associated factors. Scaling and root planing is the standard of care for nonsurgical and nonpharmacologic treatment of chronic periodontal diseases. Clinical trials have consistently demonstrated that scaling and root planing reduce gingival inflammation and probing depths and result in gains of clinical attachment in most periodontal patients.13 There are also secondary influences on periodontal health that must be considered. Calculus, although not an etiologic agent in itself, is virtually always associated with plaque biofilm, and its removal is associated with improved periodontal health.
Anatomic and iatrogenic plaque traps, such as overhanging restorations and malposed teeth, must be considered during nonsurgical therapy. Although these features are primarily plaque biofilm control problems, the dental hygienist should recognize them, design specific plaque control measures, and refer patients for further treatment. Replacement restorations or orthodontic movement of the teeth can simplify plaque biofilm control and help the patient achieve periodontal health. These local factors are described in Chapter 5.
Removal of Causative Factors
Plaque Biofilm
Plaque biofilm is the primary causative agent in gingival and periodontal diseases. Animal studies provide strong evidence that these destructive diseases occur in the presence of microbes, but not in animals raised in germ-free environments. Although some periodontal destruction has been observed in germ-free (gnotobiotic) animal experiments, it tends to be localized and related to the impaction of foreign objects, such as hairs. Inflammation and tissue destruction in conventionally raised animals with oral biota are vastly more widespread and severe.5
Bacteria live in the mouth and are present around diseased teeth. Convincing experimental evidence that plaque microorganisms cause human gingival disease was presented by Löe and colleagues in 1965.14 The researchers initiated extensive plaque control in a small group of dental students and brought them to a level of excellent periodontal health; then the subjects refrained from oral hygiene procedures for 3 weeks. Within 10 to 21 days, every subject had gingivitis, which resolved in about 1 week when oral hygiene practices were resumed. In addition, the microbial composition of dental plaque changed from one of gram-positive microbiota to one dominated by gram-negative organisms.
As the understanding of plaque biofilm as the pathologic agent has grown, various periodontal diseases have been identified with specific microbial organisms. All plaques are no longer considered intrinsically bad. The specific plaque hypothesis was proposed by Loesche in the 1970s.15 This classic study has increased the understanding of periodontal disease and the use of appropriate antimicrobial agents to improve treatment results. An excellent example of the application of the specific plaque hypothesis is the treatment of aggressive periodontitis in its juvenile form. In the 1960s, this disease was recognized as different from typical periodontitis because the conventional therapy, which consisted of scaling and root planing in the localized affected areas of the anterior teeth and first molars, could only slow the loss of these teeth. A specific plaque bacterium, Actinobacillus actinomycetemcomitans, was identified in these lesions. Therefore, treatment emphasis changed to include both conventional therapy and the use of appropriate antibiotics and resulted in successful restoration of periodontal health with less tooth loss.
Periodontal diseases present similar symptoms, but they likely have different bacterial origins that are not yet fully defined. Eventually, they will be much better understood so that therapies directed toward the specific plaque bacteria in each individual can be used, including the use of more antimicrobial and antiseptic agents.16
Although more specific gingival and periodontal diseases are recognized, nonsurgical periodontal therapy focuses on total plaque biofilm removal. The quality of the plaque is more important than the quantity, but plaque biofilm is still the causative agent in disease. Bacteria-specific tests and treatments have been developed and will be more widely used as the understanding of periodontal disease increases.7
It is possible to remove all supragingival plaque effectively. To do so, the patient uses oral hygiene procedures and the dental hygienist performs coronal polishing. However, subgingival plaque is not effectively altered by supragingival oral hygiene procedures, especially in deeper pockets of 5 mm or more. Supragingival oral hygiene procedures have limited effects on symptoms associated with deeper pockets, such as bleeding on probing.17
Subgingival plaque biofilm removal is essential in nonsurgical therapy to disrupt the established colonies of bacteria and let a younger plaque develop that is less associated with pathologic conditions. Dental hygiene procedures with hand instruments or powered scalers adequately accomplish subgingival plaque biofilm removal. A study published in the 1980s compared the performance of hand instruments with that of ultrasonic tips in the removal of plaque in pockets. Both were effective in removing approximately 67% of the plaque in pockets deeper than 5 mm and the ultrasonic instruments performed as well as the hand instruments.16,17 The AAP consensus report on nonsurgical periodontal therapy suggested that 11% plaque remaining on root surfaces after thorough instrumentation was more likely an accurate figure.17
Calculus
Calculus is little more than calcified plaque biofilm. As plaque biofilm ages, the organic matrix and bacterial cells calcify. Calculus adheres to tooth surfaces through pellicular attachment, mechanical locking, and intercrystalline forces. It varies in crystal composition, type of attachment, and degree of difficulty in removal (see Chapter 5). Although calculus is an inert substance, its role appears to be that of plaque biofilm retention, and its removal is associated with a return to periodontal health, as seen in Figure 13-4.
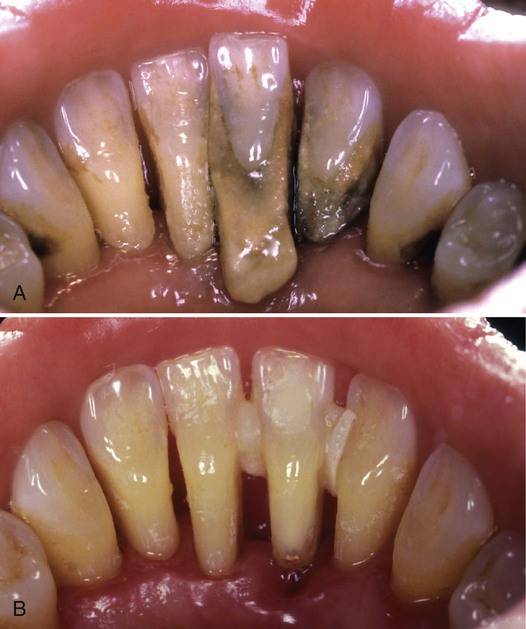
A, Pretreatment documentation for a 45-year-old woman with severe periodontal destruction and calculus accumulation. B, Periodontal healing 4 weeks after nonsurgical periodontal treatment. Note the temporary splint placed to limit mobility of teeth #24, #25, and #26.
The thoroughness of calculus removal by instrumentation has been studied and shows surprising results. Kepic and colleagues18 demonstrated residual calculus on most teeth after 45 to 60 minutes of treatment time per quadrant. Even when teeth were instrumented for as long as 39 minutes each, residual calculus was noted regularly in deeper pockets, and totally clean surfaces were achieved only in the 3- to 4-mm range.19,20 Even the best instrumentation techniques leave some residual deposits on the teeth; however, these very small deposits were also present in the subjects of long-term studies used to verify the effectiveness of nonsurgical periodontal treatment, and they did not appear to cause the treatment to fail.2,3
Root Smoothness
Achieving root smoothness is important for evaluating short-term goals during treatment appointments. The dental hygienist must develop a tactile sense that permits detection of obvious calculus on the teeth. This tactile sense is used to determine the amount of calculus present in the untreated patient, the existence of irritating factors such as overhangs, and the point at which thorough instrumentation (periodontal debridement) is finished at each appointment. Glassy, smooth root surfaces are not end points in treatment. After instrumentation, some roots feel smooth, whereas others have varying degrees of granular roughness. Experience suggests that the roots in an individual patient’s mouth will feel equally smooth after thorough instrumentation. This uniform smoothness should be identified. Most importantly, no surfaces should feel rough, as if calculus is still present. Plaque biofilm must also be dislodged from all accessible surfaces. Clearly, this requires clinical experience and judgment on the part of the dental hygienist. Armitage reviewed the reasons dental hygienists and dentists attempt to smooth roots to a glassy, hard texture through root planing. These reasons are8 as follows:
• Smooth surfaces are easier to clean.
• Smooth surfaces retard plaque formation.
Armitage presented the following information regarding root surface roughness8:
1. Slightly rough root surfaces, those that are scaled and cleaned but not planed in a systematic way to remove cementum and leave glassy surfaces, do not accumulate plaque more rapidly than smoother surfaces. The appealing notion that rough surfaces would present more of a plaque control problem for patients is borne out by experience with obvious calculus or overhanging restorations. However, the roughness associated with calculus and poor restorations is far greater than the slightly granular texture of calculus-free root surfaces.
2. Studies evaluating plaque biofilm formation on rough root surfaces are equivocal. Early studies that used visual appraisal of deposits or colony counts on surfaces showed that smooth surfaces had less plaque biofilm formation; however, root texture was not measured. The only study that attempted to measure root texture with quantifiable profilometer (Micrometrical Manufacturing, Ann Arbor, MI) readings found that the amount of root roughness did not affect plaque biofilm formation.
3. No experimental evidence indicates that rough root surfaces are mechanical irritants and would therefore delay healing.
4. Smooth root surfaces do not appear to promote better or faster healing than rough surfaces. In fact, in some studies, gingivae next to root surfaces that were notched for orientation of researchers after tooth extraction healed uneventfully in the mouth. This indicated that roughness itself had no effect on wound healing.
Root roughness has been equated with incomplete instrumentation because of concerns that endotoxins (e.g., lipopolysaccharides) formed by gram-negative bacteria invade the root structure. Removal of endotoxins would require the planing away of diseased cementum. This practice supports the old notion of “necrotic” root surfaces. Much has been learned about the penetration and removal of lipopolysaccharide endotoxins. Studies indicate that endotoxins do not penetrate deeply into cemental surfaces and that retained toxins are associated with missed calculus and plaque rather than diseased cementum. Nyman and colleagues20 compared these treatment strategies by testing the healing of quadrants after periodontal surgery. One side was treated with conventional root planing and the other with calculus carefully flicked off and root surfaces polished before the tissue was sutured back in place. There was no difference in the healing of the differently treated areas; cementum removal through root planing did not improve healing beyond that achieved by calculus removal and polishing.
These data indicate that toxins are superficially located on root surfaces and easily removed. Extensive root instrumentation is not required beyond the removal of calculus and plaque. Thus, the rationale for root planing to remove root roughness and achieve glassy, smooth root surfaces is no longer valid. Dramatically thinned root surfaces are shown in Figure 13-5. This thinning is an example of overinstrumentation or root planing without rationale.
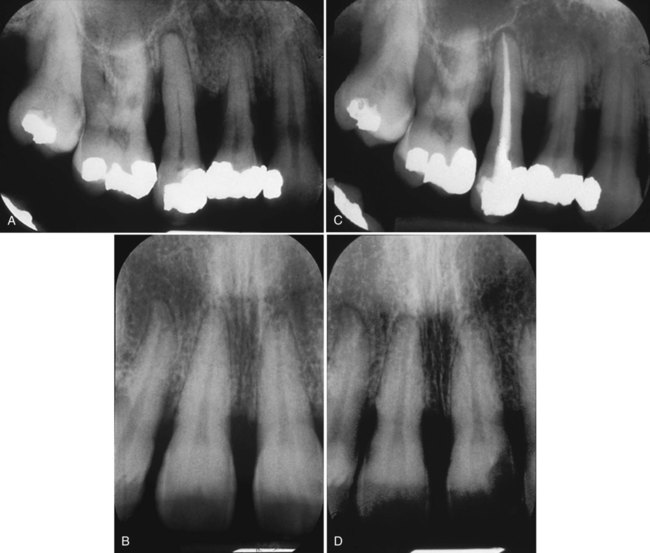
A and B, Pretreatment radiographs of periodontally involved teeth. C and D, The same teeth 3 years later, after periodontal therapy and 11 maintenance visits. The roots have been planed to a substantially reduced dimension. This is an example of excessive root planing during the maintenance phase. (Courtesy of Dr. Thomas Bramanti.)
Gingival Curettage
Gingival curettage, also called closed curettage or nonsurgical gingival curettage (truly a misnomer), was traditionally performed to remove inflamed pocket lining for reasons distinct from periodontal debridement. Inflamed pocket lining is composed of thin ulcerated strands of epithelium, with rete pegs extending into the underlying connective tissue and granulation tissue containing disorganized masses of cells. It may also contain dislodged calculus and plaque bacteria. Removal of this tissue was assumed to enhance pocket reduction beyond the results achieved by scaling and root planing alone, providing faster healing and the formation of new connective tissue attachments to the root surfaces. This rationale has been questioned for many years and the procedure is no longer considered standard treatment.21,22
No clinical studies have shown greater pocket reduction, more rapid healing, or more new attachment after gingival curettage has been performed compared with scaling and root planing alone.22 In animal studies, gingival curettage promoted the formation of long junctional epithelium during healing, rather than new connective tissue attachment.23 Clinical trials reviewed by Kalkwarf22 indicated that tissue healed through long junctional epithelium rather than connective tissue attachment can be maintained successfully for years, suggesting that this is a satisfactory treatment result.
A number of dental hygiene programs in the United States teach gingival curettage because it is a legally sanctioned duty in many states and may be performed by practitioners in the community.24 In this era of increased emphasis on nonsurgical therapies, removal of disorganized granulation tissue and ulcerated epithelium from pocket linings remains appealing to many clinicians, even if data do not show improved healing.
Healing
Soft Tissue Healing After Scaling and Periodontal Debridement
When the junctional epithelium has been injured or separated from the tooth surfaces, as it would be during periodontal debridement, healing can be expected to take approximately 1 week. Animal studies show that hemidesmosomes begin to reattach from the apical end of the junctional epithelium and are intact after 7 days. Normal turnover of cells in the junctional epithelium, which migrate from the apical end to the coronal end, takes about 5 days. Repair after disruption of the junctional epithelium during scaling procedures (not removal, which occurs with surgical excision) is similar to the normal course of events in tissue turnover.25
Inflammatory activity occurs in the underlying connective tissue during the disease process and is also a result of treatment. Connective tissue fibers are disrupted and lysed beneath the epithelium. Healing of inflamed connective tissue is complex, requiring many cells and mediators. It takes considerably longer than healing of epithelium—up to several months.26 New connective tissue fiber attachment to the tooth surface is not a predictable outcome, but the development of an elongated junctional epithelial attachment may result in reduced probe readings. Because of the fragile state of healing connective tissues, probing after treatment should be avoided for 4 weeks.17
Repopulation of Microorganisms After Therapy
Scaling and periodontal debridement are effective in reducing the volume of plaque biofilm bacteria in treated sites. The numbers of organisms are reduced dramatically and grow back in different proportions. The bacterial plaque shifts from predominantly gram-negative microbiota to one that is gram-positive, with many fewer motile forms, especially spirochetes. These new microbiota are similar to those found in periodontally healthy sites. Bacteria repopulate in a specific order, starting with Streptococcus and Actinobacillus species, followed by Veillonella, Bacteroides, Porphyromonas, Prevotella, and Fusobacterium species. Capnocytophaga species and spirochetes are the last to grow back. The cycle may take as long as 6 months to complete.8 Repopulation can be expected to vary for many reasons, one of which is clinician differences in complete removal of plaque biofilm and calculus.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses