Chapter 13. Marginal periodontitis and the dental pulp
I. Rotstein and J.H. Simon
CHAPTER CONTENTS
Summary231
Anatomical considerations231
Effect of inflamed pulp on the periodontium232
Effect of marginal periodontitis on the pulp233
Learning outcomes248
References250
SUMMARY
Endodontic-periodontal diseases often present challenges to the clinician in their diagnosis, treatment and prognosis assessment. Aetiological factors such as microorganisms, as well as contributing factors such as trauma, root resorption, perforation and dental malformation play a role in the development and progression of such diseases. Treatment and prognosis of endodontic-periodontal diseases vary and are dependent on the aetiology, pathogenesis and correct recognition of each specific condition. Therefore, understanding the interrelationship between endodontic and periodontal diseases will enhance the clinician’s ability to establish correct diagnosis, assess the prognosis of the teeth involved and select a treatment plan based on biological and clinical evidence.
ANATOMICAL CONSIDERATIONS
The dental pulp and the periodontium are intimately related and connected via exposed dentinal tubules, lateral or accessory canals, and the apical foramen.1.2.3.4.5.6.7.8.9.10.11.12.13. and 14. Exposed dentinal tubules in areas devoid of cementum may serve as viable communication pathways between the dental pulp and periodontal ligament. Exposure of dentinal tubules may occur due to developmental defects, disease and following periodontal or surgical procedures. Radicular dentinal tubules extend from the pulp to the cemento-dentinal junction (CDJ). They run a relatively straight course and range in size from 1 to 3 µm in diameter. 13 The diameter of the tubules decreases with age or as a response to chronic low grade stimuli causing apposition of highly mineralized peritubular dentine. The number of dentinal tubules varies from approximately 8000 mm−2 at the CDJ to 57 000 mm−2 at the pulpal end. In the cervical area of the root the number of dentinal tubules is about 15 000 mm−2. 13
When the cementum and enamel do not meet at the cemento-enamel junction (CEJ) these tubules remain exposed thereby creating pathways of communication between the pulp and the periodontal ligament. Patients experiencing cervical dentine hypersensitivity are an example of such a phenomenon. Fluid and irritants may flow through patent dentinal tubules. In the absence of an intact enamel or cementum layer, the pulp can become exposed to the oral environment via the gingival sulcus or periodontal pocket. Experimental studies demonstrated that soluble material from bacterial plaque applied to exposed dentine can cause pulpal inflammation indicating that dentinal tubules may provide ready access between the periodontium and the pulp. 15
Scanning electron microscopic studies have demonstrated that dentine exposure at the CEJ occurred in about 18% of teeth in general and in 25% of anterior teeth in particular. 16 In addition, the same tooth may have different CEJ characteristics presenting dentine exposure on one surface while the other surfaces are covered with cementum. 17 This area is susceptible to the progression of endodontic pathogens, as well as to the effect of root scaling and planing on cementum integrity, trauma, and bleaching-induced pathosis.18.19. and 20. Other areas of dentinal communication may be through developmental grooves both palato-gingival and apical. 21 The base of these grooves is often not covered by cementum and accessory canals are often present.
Lateral and accessory canals can be present anywhere along the length of the root. Their incidence and location have been well documented in both animal and human teeth using a variety of methods. These included dye perfusion, injection of impression materials, microradiography, light microscopy and scanning electron microscopy.2.4.6.8.10.14. and 22. It is estimated that 30–40% of all teeth have other smaller canal systems and the majority of them are found in the apical third of the root. It was reported that 17% of teeth presented multiple canal systems in the apical third of the root, about 9% in the middle third, and less than 2% in the coronal third. 4 However, it seems that the incidence of periodontal disease associated with these types of canals is relatively low. A study of 1000 human teeth with extensive periodontal disease found only 2% of such canals associated with the involved periodontal pocket. 8
Other canal systems in the furcation of molars may also be a direct pathway of communication between the pulp and the periodontium. 6,10 The incidence of accessory canals may vary from 23% to 76%.2.4. and 23. These accessory canals contain connective tissue and blood vessels that connect the circulatory system of the pulp to that of the periodontium. However, not all these canals extend the full length from the pulp chamber to the floor of the furcation. 23 It was reported that pulpal inflammation may cause inflammatory reaction in the interradicular periodontal tissues. 24 The presence of these patent smaller canals is a potential pathway for the spread of microorganisms and their toxic byproducts from the pulp to the periodontal ligament and vice-versa, resulting in an inflammatory process in the involved tissues.
The apical foramen is the principal route of communication between the pulp and periodontium. Microbial and inflammatory byproducts may exit readily through the apical foramen to cause periapical pathosis. The apex is also a potential portal of entry of inflammatory byproducts from deep periodontal pockets into the pulp. Pulp inflammation or pulp necrosis extends into the periapical tissues causing a local inflammatory response often associated with bone and root resorption. 24 Endodontic therapy aims to eliminate the intraradicular aetiological factors thereby leading to healing of the affected periapical tissues.
EFFECT OF INFLAMED PULP ON THE PERIODONTIUM
When the pulp becomes inflamed, it elicits an inflammatory response in the periodontal ligament at the apical foramen and/or adjacent to smaller openings of the root canal system. 25 Inflammatory byproducts of pulpal origin may permeate through the apex, smaller canals in the apical third of the root canal system or exposed dentinal tubules and trigger an inflammatory vascular response in the periodontium.26.27.28.29.30.31.32. and 33. Among those are living pathogens such as certain bacteria strains including spirochetes, fungi and viruses,26.27.29.33.34.35.36.37. and 38. as well as non-living pathogens.37.39.40.41. and 42. Many of these are pathogens similarly encountered in periodontal inflammatory disease. In certain cases, pulpal disease will stimulate epithelial growth affecting the integrity of the periapical tissues. 43,44
In an experimental study, defects of different sizes were created on root surfaces of extracted lateral incisors with open or mature apices. The canals were either infected or filled with calcium hydroxide and then replanted. It was observed that intrapulpal infection promoted marginal epithelial downgrowth on the denuded dentine surface after 20 weeks. 45 The effects of endodontic pathogens on marginal periodontal wound healing on dentinal surfaces surrounded by healthy periodontal ligament have been assessed. 46 It was found that in infected teeth the defect was covered by 20% more epithelium and the non-infected had 10% more connective tissue covering. It appears that the pathogens in a necrotic canal can stimulate epithelial downgrowth along denuded dentine surfaces with marginal communication.
The effect of endodontic infection on periodontal probing depth and the presence of furcation involvement in mandibular molars have also been investigated. 47 In 100 patients with molars with periapical lesions on both roots, the periodontal probing depth was significantly greater than around teeth without periapical lesions. It was suggested that root canal inflammation in molars involved with marginal periodontitis may potentiate periodontitis progression by pathogens spreading through accessory canals and dentinal tubules causing more attachment loss in the furcation.
Periodontal pathogens in pulp and periodontal diseases affecting the same tooth were studied by means of 16S RNA gene directed polymerase chain reaction samples from 31 teeth. 48 Specific polymerase chain reaction methods were used to detect Actinobacillus actinomycetemcomitans, Bacteroides forsythicus, Eikenella corrodens, Fusobacterium nucleatum, Porphyromonas gingivalis Prevotella intermedia and Treponena denticola. The pathogens were found in all endodontic samples. In chronic apical periodontitis and chronic marginal periodontitis the same pathogens were found. It was concluded that periodontal pathogens often accompany endodontic infections and support the concept that endodontic-periodontal interrelationships are a critical pathway for both diseases. In addition, foreign bodies and materials may also pass into the periapical tissues. Extrinsic foreign bodies, including foreign lipids, cellulose granulomas and iatrogenic materials, can cause a direct inflammatory response.
EFFECT OF MARGINAL PERIODONTITIS ON THE PULP
The effect of periodontal inflammation on the pulp is controversial and conflicting studies abound.3.5.12.24.49.51.52.53.55.57. and 58. It has been suggested that marginal periodontitis has no effect on the pulp, at least, until it involves the apex. 3 On the other hand, several studies suggested that the effect of periodontal disease on the pulp is degenerative in nature including an increase in calcifications, fibrosis and collagen resorption, as well as a direct inflammatory effect. 9,11 It appears that the pulp is usually not severely affected by periodontal disease until recession has opened an accessory canal to the oral environment. At that stage, pathogens leaking from the oral cavity through the accessory canal into the pulp may cause a chronic inflammatory reaction followed by pulpal necrosis. However, as long as the accessory canals are protected by sound cementum, necrosis usually does not occur. Additionally, if the mircovasculature of the apical foramen remains intact the pulp will maintain its vitality. 9 With regard to the root apex, once the apical vasculature is compromised, the pulp will lose its vitality. This is shown in teeth with primary periodontal lesions with secondary endodontic involvement (see later). The effect of periodontal therapy on the pulp is similar during scaling, curettage or periodontal surgery if accessory canals are opened to the oral environment. In such cases, pathogenic invasion and secondary inflammation and necrosis of the pulp can occur.
CLASSIFICATION
There are many ways of classifying the so-called endodontic-periodontal (endo-perio) lesions.24.52.59.60. and 61. For differential diagnostic and treatment purposes they are best classified as endodontic, periodontal or combined diseases. 62,63 They include:
1. primary endodontic lesion
2. primary periodontal lesion
3. combined lesions.
The combined lesions include:
1. primary endodontic lesion with secondary periodontal involvement
2. primary periodontal lesion with secondary endodontic involvement
3. true combined lesions.
This classification is based on the theoretical pathways explaining how these lesions are formed. 61 By understanding the pathogenesis, the clinician can then suggest an appropriate course of treatment and better assess the prognosis.
Primary endodontic lesion
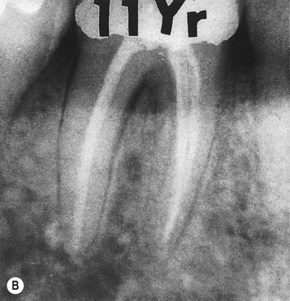
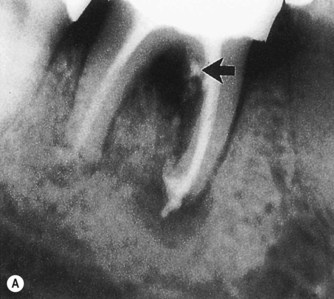
An acute exacerbation of a chronic periapical lesion on a tooth with a necrotic pulp may drain coronally through the periodontal ligament into the gingival sulcus (Fig. 13.1A). This condition may mimic a periodontal abscess. However, it is only periodontal in that it passes through the periodontal ligament space (Figure 13.2, Figure 13.3, Figure 13.4 and Figure 13.5). In reality, it is a sinus tract resulting from pulpal disease. Therefore, it is essential that a gutta-percha cone is inserted into the sinus tract and one or more radiographs taken to track the origin of the lesion. When the sinus tract is probed, it is usually narrow and lacks width. A similar situation occurs where drainage from the apex of a molar tooth extends coronally into the furcation area (Fig. 13.6). Direct extension of inflammation from the pulp may also occur into the furcation area of a non-vital tooth when a lateral or accessory canal is present (Fig. 13.7). Primary endodontic lesions usually heal following root canal treatment; the sinus tract extending into the gingival sulcus or furcation disappears at an early stage (usually within a few weeks) once the consequences of the necrotic pulp has been treated. It is important to recognize that an attempt to provide periodontal therapy for this condition will result in failure since if the necrotic pulp has not been diagnosed and treated.
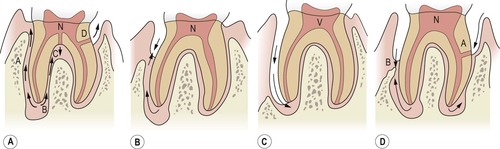 |
| Figure 13.1
Classification of endodontic-periodontal lesions. (A) Primary endodontic lesions: pathway extending from apex to gingival sulcus via periodontium (A); apex to furcation (B); lateral canal to furcation (C); lateral canal to pocket (D). (B) Primary endodontic lesion with secondary periodontal involvement. (C) Primary periodontal lesion extending to the apex. (D) Primary periodontal lesion with secondary endodontic involvement via a lateral canal (A). Combined lesion from coalescence of separate lesions (B). N = Necrotic pulp. V = Vital pulp.
|
 |
| Figure 13.2
Primary endodontic lesion. Mandibular premolar with a radiolucency along the distal surface of the root. (A) Pretreatment, the lesion (arrowed) drained through the gingival sulcus. (B) Immediately after treatment, root canal sealer can be observed in the sinus tract. (C) Six months later, there is evidence of healing and bone filling.
|
 |
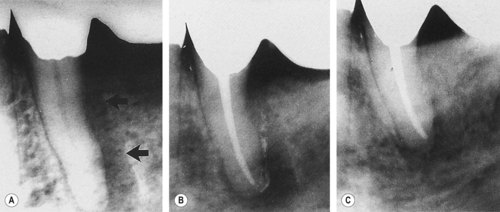
| Figure 13.3
(A) Mandibular molar with a narrow probable distal pocket and large radiolucency (arrowed). (B) Marked healing apparent at one-year recall confirming that the original ‘pocket’ was of endodontic origin.
|
 |
| Figure 13.4
Success following treatment of a primary endodontic lesion. (A) Immediate post-treatment radiograph of a mandibular canine showing mesial radiolucency (arrowed). (B) Radiograph at 15 months showing healing.
|
 |
| Figure 13.5
Mandibular molar with apical lesion extending into furcation. (A) Preoperative radiograph showing furcal and distal radiolucency. (B) Clinical photograph of gingival swelling and a periodontal probe in the furcation. (C) A 1-year recall radiograph showing furcal and distal radiolucencies healing. (D) Clinical photograph showing minimal pocket depth on the buccal. Healing occurred following root canal treatment alone.
|
 |
 |
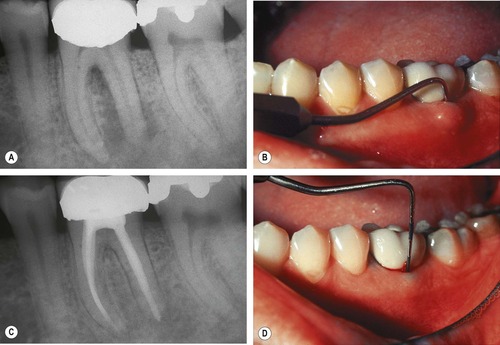
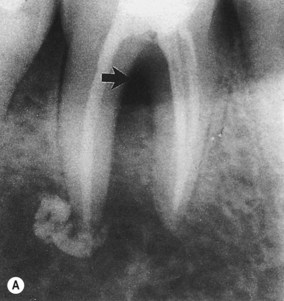
| Figure 13.6
Mandibular molar where apical involvement extends into the furcation (arrowed). (A) Immediately following root canal treatment, excess sealer is present at the apex of the distal root. (B) Radiograph 11 years later showing apical and furcation healing.
|
 |
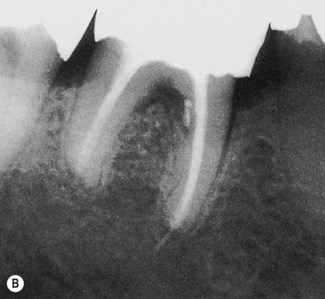 |
| Figure 13.7
Primary endodontic lesion with a lateral canal demonstrated in the furcation. (A) Post-treatment radiograph demonstrating filling material passing through openings into the furcation (arrowed). (B) After 18 months, complete healing of the lesions at the apex and adjacent to the lateral canal is demonstrated.
|
Primary periodontal lesion
These lesions (Fig. 13.1C) are caused by marginal periodontitis, which progresses apically along the root surface until the apical region is reached. In such conditions, pulp sensitivity testing will reveal a clinically normal pulpal response (Figs 13.8& 13.9). In addition, a probable pocket that has width is anticipated, possibly becoming progressively shallower as the probe is moved laterally; there is also an accumulation of plaque and frequently calculus. The prognosis in this condition depends wholly upon the stage of the marginal periodontitis and the efficacy of periodontal therapy. The clinician must also be aware of the radiographic appearance of marginal periodontitis associated with developmental radicular anomalies (see later).
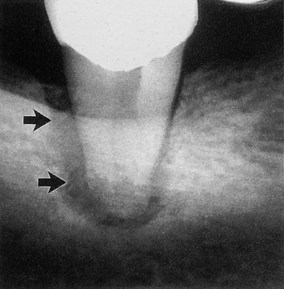 |
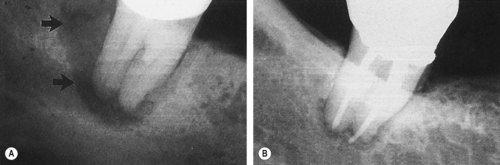
| Figure 13.8
Primary periodontal lesion. A mandibular molar presented with a probable distal pocket (arrowed). Pulp testing was positive indicating a lesion of periodontal origin; the tooth was extracted.
|
 |
 |
 |
 |
 |
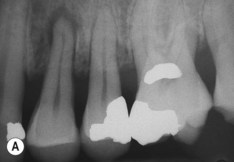
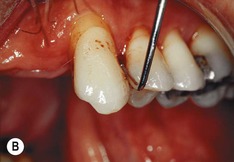
| Figure 13.9
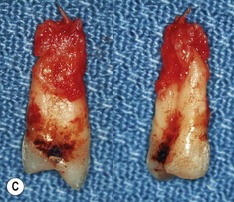
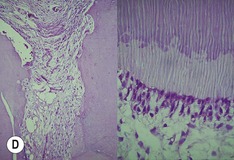
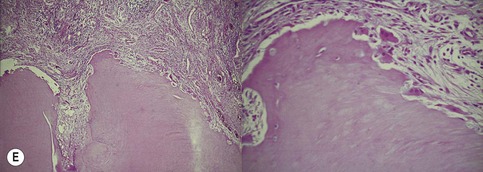
Primary periodontal lesion. (A) Preoperative radiograph of maxillary first premolar with a periapical lesion but no obvious endodontic aetiology. (B) Clinical photograph with periodontal probe in place. The pulp will respond normally to pulp sensitivity testing. (C) Two views of the extracted tooth. Note mesial anatomical groove along the root. (D) Micrographs showing vital pulp in the tooth. (E) Micrographs showing inflammatory resorption and periodontitis.
|
Combined lesions
Primary endodontic lesion with secondary periodontal involvement
If, after a period of time, a suppurating primary endodontic lesion remains untreated, it may become secondarily involved with marginal periodontal breakdown (Fig. 13.1B). Biofilm forms at the gingival margin of the sinus tract and leads to marginal periodontitis. When plaque or calculus is encountered upon probing, the treatment and prognosis of the tooth are altered; the tooth now requires both endodontic and periodontal treatments. If the endodontic therapy is adequate, the prognosis depends on the severity of the marginal periodontal damage and the efficacy of periodontal therapy. With endodontic therapy alone, only part of the lesion will heal to the level of the secondary periodontal lesion.
Primary endodontic lesions with secondary periodontal involvement may also occur as a result of root perforation during root canal treatment, or where pins or posts have been misplaced during coronal restoration (Fig. 13.10). Symptoms may be acute, with periodontal abscess formation associated with pain, swelling, exudation of pus, pocket formation and tooth mobility. A more chronic response may sometimes occur without pain, and involves the sudden appearance of a pocket with bleeding on probing or exudation of pus. When the root perforation is situated close to the alveolar crest, it may be feasible to raise a flap and repair the defect with an appropriate filling material and subsequently reposition the flap apically to expose the repaired perforation. In deeper perforations, or in the roof of the furcation, immediate internal repair of the perforation has a better prognosis than management of an infected one. Many materials have been used for this purpose. Today, Mineral Trioxide Aggregate is most widely used. 64,65
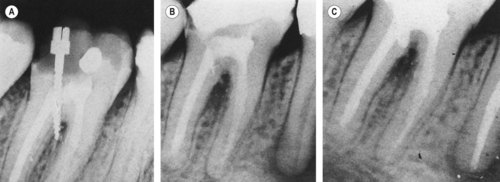 |
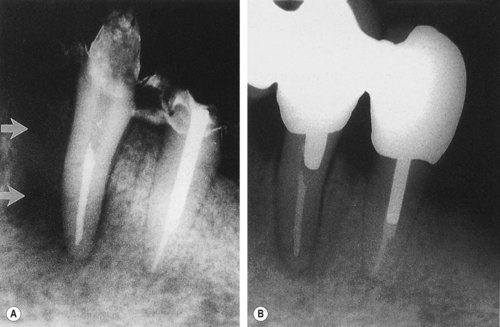
| Figure 13.10
Primary endodontic lesion with secondary periodontal involvement. (A) A screw post has perforated the furcation area of a mandibular second molar. (B) After 6 months, furcation bone loss is evident. (C) The perforation has been treated from within the tooth and the pocket has been curetted. Some new bone has formed in the furcation and clinically, no pocket can be probed.
|
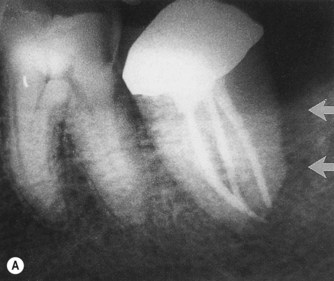
Root fractures may also present as primary endodontic lesions with secondary periodontal involvement. These typically occur on root-treated teeth (Fig. 13.11), often with post crowns in situ. The signs may range from a local deepening of a periodontal pocket to more acute periodontal abscess formation. In addition, root fractures have become an increasing problem with molar teeth that have been treated by root resection. In a study of 100 patients, a total of 38 teeth failed during the 10-year period of observation, and 47% of the failures were due to root fractures, with the vast majority being in mandibular molar teeth. 54,56
 |
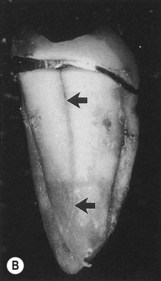 |
| Figure 13.11
Longitudinal fracture. (A) A distal pocket (arrowed) probable to the apex on a mandibular left second molar persisted following root canal treatment, necessitating extraction. (B) Following extraction, a longitudinal fracture on the distal root surface (arrowed) was evident extending the length of the tooth. Fused roots prevented ‘anatomical redesigning’.
|
Primary periodontal lesion with secondary endodontic involvement
The apical progression of a periodontal pocket can continue until the apex is reached. As a result, the pulp may become necrotic due to irritants permeating via a lateral canal or the apical foramen (Fig. 13.1D). In single rooted tooth the prognosis is usually poor, which is the opposite of that for the primary endodontic lesion. In molar teeth not all the roots may suffer the same loss of supporting tissues to the apex, in which case the possibility of root resection should be considered.
The treatment of marginal periodontitis can also lead to secondary endodontic involvement. Lateral or accessory canals and dentinal tubules can be opened to the oral environment by curettage, scaling or surgical flap procedures (Figs 13.12& 13.13). In addition, it is possible for a blood vessel within a lateral canal to be severed by a curette during treatment. Also, pulp changes resulting from marginal periodontitis were observed when the main apical foramen was involved. 9 Provided the blood supply through the apex is intact, the pulp has a strong capacity for survival (Fig. 13.10).



