Chapter 10 Orofacial viral infections
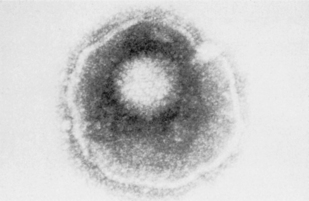
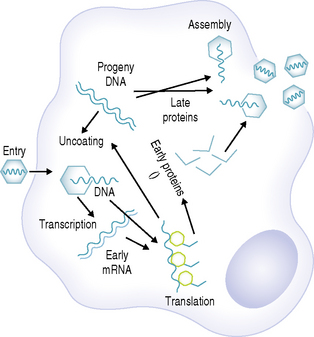
Viruses, which are some of the smallest microorganisms (100–300 nm), consist of a core (genome) containing either DNA or RNA surrounded by a protein shell (capsid) (Fig. 10.1), can cause a range of important human diseases. Certain viruses also possess an outer lipoprotein coat derived from infected host cells. Viruses are obligate intracellular parasites in that they require the protein synthesizing apparatus (ribosomes) of the host cell. Viral replication is a complex process but comprises a number of recognized steps including, adsorption/penetration into the host cell, uncoating, transcription, synthesis of viral components, assembly and finally, release of new virions (Fig. 10.2).
Antiviral agents
The development of aciclovir was a milestone in antiviral therapy, representing the first true specific antiviral agent, recognized by the award of the Nobel Prize for Medicine. Aciclovir is a nucleoside analogue drug that has activity against members of the herpes group of viruses, in particular herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2). Viral enzymes, within HSV-infected cells, phosphorylate aciclovir to monophosphate and the agent becomes cell bound. Subsequent further phosphorylation to aciclovir triphosphate produces an analogue to deoxyguanosine triphosphate that inhibits viral DNA synthesis and prevents further viral replication.
Laboratory diagnosis
A variety of laboratory methods have been developed for the detection, isolation and identification of viruses within clinical samples (Table 10.1). These techniques involve microscopy, culture, serology and nucleic acid amplification. However, the most appropriate method of sampling depends on the nature of the suspected infection.
Table 10.1 Special investigations used in the diagnosis of orofacial viral infection.
| Investigation | For | Against |
|---|---|---|
| Electron microscopy of vesicular fluid | Easy sampleRapid result | InsensitiveNot specific |
| Light microscopy of lesional smear | Easy sample of smear Widely availableRapid result | Not specific |
| Light microscopy of lesional smear with immunofluorescence | Easy sampleRapid resultSpecific | Not routinely available |
| Culture of swab of lesion | Easy sampleCan be specificWidely available | Result may not be available for up to 10 days |
| Antibody titre in venous blood | Requires paired venous samples | Two haematological samples required.Result not available for at least two weeks |
| PCR amplification of vesicular fluid or swab of lesion | Easy sampleRapid result | Not widely available |
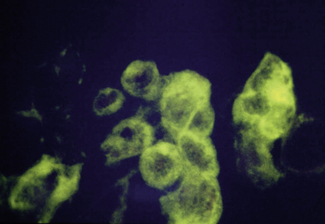
Electron microscopy can be used to provide a provisional identification based on the morphological appearance of viral particles but this approach has low specificity and requires additional tests. Routine light microscopy can be used in conjunction with immunofluoresence and monoclonal antibodies to detect the presence of specific viruses (Fig. 10.3). This is a relatively rapid method giving a result within 30 minutes but the technique is not widely available.
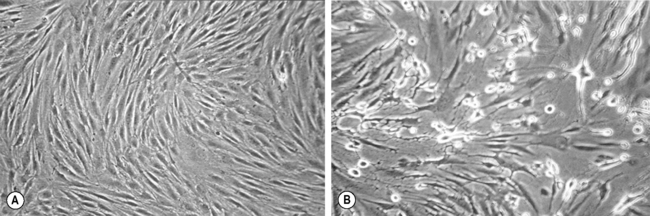
Viruses can be grown in tissue culture usually involving baby hamster or monkey kidney fibroblasts. A swab of the lesion should be placed in viral transport medium, which contains at least two antibiotics, usually penicillin and streptomycin, to prevent bacterial growth, combined with an antifungal, such as amphotericin, to eliminate any fungal contamination. The transport medium should also contain serum and a buffer to maintain virus viability. The specimen should be sent to the laboratory promptly although it will not be processed for 24 hours to allow the antimicrobials mentioned above time to have an effect. The first stage of processing is inoculation into a monolayer of tissue culture. The presence of virus is determined in the laboratory by detection of a cytopathic effect, which is seen as the development of multinucleate giant cells (Fig. 10.4). This may take up to 10 days although the cytopathic effect occurs more rapidly in the presence of high numbers of virus particles.
Herpes viruses
The name herpes comes from the Greek word ‘herpein’ which means to creep (chronic, recurrent). While more than 100 herpes viruses have been isolated in nature, only eight herpes viruses have been described in humans and these are classified according to their biological properties into three sub-families: Alphaherpesvirinae, Betaherpesvirinae and Gammaherpesvirinae (Table 10.2), all of which may be encountered in orofacial tissues.
Table 10.2 Nomenclature of human herpes viruses (Herpesviridae).
| Name | Trivial name | Acronym |
|---|---|---|
| Alphaherpesvirinae | ||
| Human herpesvirus 1 | Herpes simplex virus 1 | HSV-1 |
| Human herpesvirus 2 | Herpes simplex virus 2 | HSV-2 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses